Knowledge Center
Medical Equipment Compliance
Medical Equipment Compliance occurs when healthcare organizations complete all planned preventive maintenance activities on medical equipment on time. This can be achieved in one of two ways. The healthcare organization can be...
Read MoreInstrument Cleaning Brushes
Using the right tool for the job is important and one of the keys to effective cleaning is having the right instrument cleaning brush. This article will review how to choose the correct...Read More
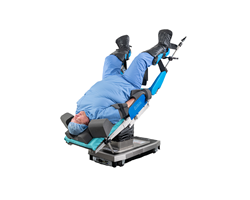
The Ultimate Guide to the Trendelenburg Position
Positioning is imperative to patient safety during a surgical procedure. Proper patient positioning depends on the type and length of procedure, anesthesia access to the patient, devices required and other factors. While in Trendelenburg position, the patient is laid supine on the surgical table, and their head is... Read MoreWhat is Sterility Assurance Monitoring?
Sterility assurance monitoring is a vital component of your overall quality assurance protocol. Sterility assurance products such as Biological Indicators (BI) and Chemical Indicators (CI) give you the confidence that the sterilizer in use is functioning properly and cycle conditions are adequate to produce medical devices and... Read MoreKnowledge Center Category List
| FOLLOW US | |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|