Knowledge Center
April 11, 2023
Guide to High-Level Disinfection of Endoscopy Devices
What Is High-Level Disinfection?
High-level disinfection, also known as HLD, is the complete elimination of all microorganisms in or on an instrument, except for small numbers of bacterial spores.1 The FDA further defines a high-level disinfectant as a sterilant used for a shorter contact time to achieve a 6-log1 kill of an appropriate Mycobacterium species.2
Before HLD is performed, devices must be properly cleaned to ensure the removal of soils. Once the device has been cleaned and rinsed, as directed in the device instructions for use (IFU), it can be high-level disinfected.
Why is High-Level Disinfection Needed?
In the broadest sense, high-level disinfection kills or inactivates microorganisms that could cause infection when used according to product labeling. Many medical devices are reusable, as single-use devices can be costly and not environmentally sustainable. Adequate reprocessing of reusable medical devices is critical in protecting patient safety. HLD inactivates microorganisms when used according to product labeling.
What are the 3 Levels of Disinfection?
While this article focuses on HLD for surgical and endoscopy devices, it is important to note that disinfection is used for various reasons around hospitals. Disinfection is critical to prevent cross-contamination or the spread of germs. The type of microorganisms, quantity, and application of the item being disinfected determine the strength of disinfectant needed and which of the three levels of disinfection should be used.
| Low-Level Disinfection |
Intermediate-Level Disinfection |
High-Level Disinfection |
|---|---|---|
| A process that kills most vegetative bacteria, some viruses, and some fungi, but not mycobacteria or bacterial spores. Low-level disinfectants are appropriate for non-critical medical devices and environmental surfaces. Examples include – stethoscopes and blood pressure cuffs. | A process that kills viruses, mycobacteria, fungi, and vegetative bacteria, but not necessarily bacterial spores. Intermediate-level disinfectants are also appropriate for non-critical medical devices, horizontal surfaces, and floors. | A process that kills all microbial organisms but not necessarily large numbers of bacterial spores. High-level disinfectants are often used for semi-critical devices. Examples include – flexible gastroscopes and bronchoscopes. |
Despite the level of disinfection needed, clinical staff must be trained to recognize what each disinfectant is designed for, how each type of device should be treated, and what level of disinfection is appropriate.
Medical devices will provide specific instructions and methods for cleaning and reprocessing as detailed in their IFUs. While some medical devices require high-level disinfection, some may require sterilization. Sterilization, such as liquid chemical sterilization for endoscopes or steam sterilization for a pair of forceps, goes one step further than high-level disinfection by aiming to kill all microbes, including bacterial endospores, on the device. While certain instruments require sterilization, other instruments, including semi-critical endoscopes, can be safely reprocessed using high-level disinfection.
Healthcare Industry Standards and Guidelines for High-Level Disinfection

While the CDC and FDA do not provide high-level disinfection policies, they do provide recommendations for healthcare facilities in the United States, which are often adopted by organizations like the Association for the Advancement of Medical Instrumentation® (AAMI), The Association of periOperative Registered Nurses (AORN) and The Society of Gastroenterology Nurses and Associates (SGNA). Outside the United States, ISO standards provide recommendations for the processing of medical devices, and some countries adopt AAMI standards as well.
ANSI/AAMI ST91, the Comprehensive Guide to Flexible and Semi-Rigid Endoscope Processing in healthcare facilities provides sterilization and high-level disinfection guidelines of flexible endoscopes. ST91 includes guidance for the disinfection of most semi-critical endoscopes, such as colonoscopes and gastroscopes. In some cases, for very complex endoscopes, such as ureteroscopes and cystoscopes that are deemed high-risk, ST91 recommends liquid chemical sterilization (LCS).
What Items Require High-Level Disinfection?
Many departments within the healthcare system rely on HLD. First, it is important to understand the difference between critical, semi-critical, and non-critical devices.
Critical Devices
Critical devices are typically used for invasive procedures and present the highest risk as they enter a "sterile" area of the body, such as the bloodstream. Common critical devices include surgical instruments, implanted medical devices, and laparoscopes. Sterilization of these devices is required.
Semi-Critical Devices
Semi-critical devices pose a lower risk when compared to critical devices, as they may only contact mucous membranes or non-intact skin. If sterilization is not available, HLD is the minimum level accepted for these devices. Other devices outside of endoscopy that require HLD include bronchoscopes, dilators, ultrasound devices, and some accessories used in surgical and endoscopy procedures.
Non-Critical Devices
Non-critical devices present the lowest risk to patients, as they may only contact intact skin. Common non-critical devices include stethoscopes, blood pressure cuffs, and bedpans. Low or intermediate-level disinfection is often recommended.
Manual vs. Automated High-Level Disinfection
High-level disinfection can be achieved with a manual or automated process. A manual high-level disinfection process typically involves soaking a device for a period of time within a specific temperature and concentration. However, per ANSI/AAMI ST91:2021, it is not recommended to use a manual process as this increases the chance of variability and inconsistency in the disinfection process. Automated processes provide more efficient, consistent, and measurable outcomes and ensure less exposure to staff.
High-Level Disinfection of Endoscopes
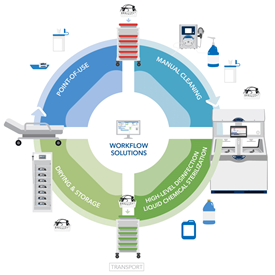
High-level disinfection is a critical step in reprocessing endoscopes to ensure they are safe for reuse. The process of high-level disinfection involves the following steps:
- Manual cleaning prior to HLD: To prepare for disinfection, endoscopes must be thoroughly cleaned of organic debris, including blood and bodily fluids.
- HLD or LCS: The endoscope is immersed in a high-level disinfectant solution for the recommended amount of time. It is recommended that high-risk devices should be sterilized. An example would be to use a LCS processor to ensure all microorganisms, including bacterial endospores, have been removed.
- Rinsing: After disinfection, the endoscope must be thoroughly rinsed to remove residual disinfectant.
- Drying: The endoscope should be dried to help prevent bacterial proliferation.
- Storage: The endoscope should be stored in a cabinet that circulates HEPA-filtered air through the cabinet or channels, until it is used again.
If it isn’t clean – it can’t be high-level disinfected or sterilized.
It is important to note that the specific instructions for high-level disinfection of endoscopes can vary depending on the type of endoscope and the disinfectant used. Following the manufacturer's instructions and local infection control guidelines is essential to ensure proper reprocessing and reduce the risk of healthcare-associated infections (HAI).
An Automated Endoscope Reprocessor (AER) is often used for endoscope reprocessing. After manual cleaning, the automated processing in an AER includes washing and circulating HLD solution through the device channels for a timed exposure period and rinsing the endoscope surfaces and internal channels with filtered water to remove the HLD residues.3
Benefits of using AERs for High-Level Disinfection
- Efficient: AERs provides a highly efficient means of cleaning and disinfecting endoscopes, reducing the risk of cross-contamination and increasing operational efficiency.
- Consistent Quality: AERs are programmed to follow specific protocols and provide consistent results, ensuring a HLD is achieved every time.
- Increased Safety: By automating the cleaning process, AERs reduce the risk of human error, providing a safer environment for patients and healthcare workers.
- Time Savings: AERs can significantly reduce the time required to clean and disinfect endoscopes, compared to manual processing, freeing up staff time for other tasks.
- Compliance: AERs can help healthcare facilities meet regulatory requirements for endoscope reprocessing, ensuring compliance with relevant standards and guidelines.
- Traceability: AERs often have built-in record-keeping systems, allowing for easy tracking of endoscope cleaning and disinfection processes and providing accountability and traceability.
Other devices that require High-Level Disinfection
HLD is required for medical devices that come in contact with mucous membranes or non-intact skin and pose a high risk of transmitting infectious agents.
Some examples of these devices include:
- Some Dental Handpieces
- Respiratory therapy equipment (nebulizers and ventilators)
- Anesthesia equipment (masks and breathing circuits)
- Dialysis equipment (catheters and tubing)
It is important to note that these devices should only be used after HLD has been properly carried out to reduce the risk of HAIs.
Examples of High-Level Disinfection products and processors
Effective high-level disinfection ensures the risk of infection is minimized, all while keeping your department running smoothly. Stay compliant with the latest regulations and guidelines with STERIS's high-level disinfectants and automated endoscope reprocessors.
Automated Endoscope Reprocessors (AERs):
Manual High-level Disinfectants:
Article References
1, 2 A Rational Approach to Disinfection and Sterilization, Centers for Disease Control and Prevention, September 2016. https://www.cdc.gov/infection-control/hcp/disinfection-sterilization/rational-approach.html?CDC_AAref_Val=https://www.cdc.gov/infectioncontrol/guidelines/disinfection/rational-approach.html
3 ANSI/AAMI ST91:2021 Flexible and Semi-Rigid Endoscope Processing in Healthcare Facilities, American National Standard 2022.