Knowledge Center
March 20, 2024
Guide to Sterilization of Flexible Endoscopes in Healthcare

Flexible endoscopes in healthcare are used in various medical procedures to diagnose and treat conditions such as reflux, ulcers, polyps, and other intestinal diseases. These procedures use colonoscopes, duodenoscopes, bronchoscopes, and cystoscopes, to name a few. In this article, you will learn about the following:
- Flexible endoscope reprocessing cycle
- Components & different types of flexible endoscopes
- Differences between high-level disinfection and sterilization
- What log reduction is
- Flexible Endoscopes Sterilization
- The standards and guidelines for sterilizing endoscopes
- How to Sterilize Flexible Endoscopes
What are Flexible Endoscopes?
Components and Types of Flexible Endoscopes
Flexible endoscopes are delicate, complex medical devices requiring special care, handling, and reprocessing. Typical components of a flexible endoscope include:

- Mechanical System: Control body, insertion tubes, lights, and bendable sections
- Imaging Systems: Often video fiberoptic cables, connectors, and water-resistant caps
- Channels: Suction and biopsy channels, as well as air or water and elevator guide wires
- Accessories: Valves, biopsy forceps, snares, and dilators
While many flexible endoscopes have similar components, each device type is unique and specialized, often for a specific procedure. A few examples include:
- Gastroscopes – Used to examine the upper digestive tract
- Colonoscopes – Used during colonoscopy procedures to view the large intestine
- Bronchoscopes – Used to check airways, including lungs
During a procedure, these complex endoscopes and their internal channels/lumens are exposed to various body fluids and chemistries that are transferred to surfaces and inside channels or lumens. Before the endoscope can be available for the next patient, it must undergo a series of reprocessing steps to remove microorganisms and bioburden to a level safe for that next patient.
With more than 20 million gastroenterology procedures each year1 and various procedures using flexible endoscopes expected to grow at 1.3% from 2023-20302, sterile processing and endoscopy technicians have many complex endoscopes that need reprocessing.
Flexible Endoscope Reprocessing Cycle
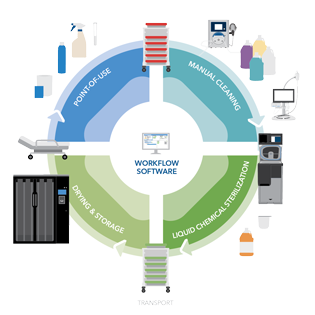
This article will focus on the options to sterilize flexible endoscopes. To learn more about the entire endoscope reprocessing cycle, read our healthcare knowledge center article What is Endoscope Reprocessing?
The endoscope reprocessing steps include:
- Point-of-Use Treatment
- Leak Testing
- Manual Cleaning and Verification
- High-Level Disinfection or Sterilization
- Endoscope Drying and Storage
- Transporting of Endoscopes
High-Level Disinfection vs. Sterilization
High-level disinfection (HLD) is the minimum standard for endoscope reprocessing; however, Liquid Chemical Sterilization provides a higher standard of care than HLD. The diagram below identifies the differences between sterilization and the 3 levels of disinfection.

*Based on the 6-log reduction of bacteria.
Proper high-level disinfection uses a germicide that, when used according to the manufacturer's instructions for use, inactivates microbial pathogens except large numbers of bacterial endospores.3 In contrast, sterilization is a validated process to render an item free from all viable microorganisms including bacterial endospores.
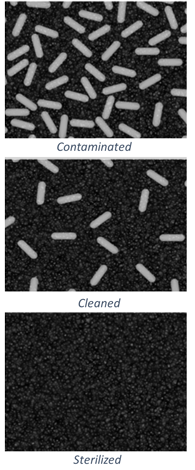
What is Log Reduction?
Studies reveal that an endoscope may carry up to 10 billion bacteria after a standard procedure4. Reprocessing is designed to reduce and remove bacteria, and the measurement of this reduction is called "log reduction." For example, a 1 log reduction means inactivating 90 percent of a target microbes.
- Manual cleaning steps can reduce bacteria load by 10^2 or up to 10^4 if all manual cleaning steps are completed perfectly
- After cleaning, HLD can reduce microbes a further 10^6 decrease
- With maximum initial contamination, average manual cleaning efficacy, and standard HLD, 100 bacteria may remain before storage4.
Sterilization is a process where all forms of viable microbial life, including bacteria, viruses, spores, and fungi, are inactivated; the term 'sterile' means free from all viable microorganisms.
Flexible Endoscopes Sterilization
ANSI/AAMI ST91 states that personnel should refer to the medical device manufacturer's written instructions for use (IFU) and the device's intended use to determine the best reprocessing method for a specific device. "Semi-critical devices come in contact with mucus membranes or non-intact skin. These [semi-critical devices] should be thoroughly cleaned and then sterilized. If sterilization is not possible, high-level disinfection is the minimum advised reprocessing method."5
During diagnostic procedures, while the intent is to inspect and identify a problem or illness, some diagnostic procedures turn therapeutic once a biopsy is needed. Now that the sterile membrane has been punctured, the best practice would be to use a sterilize device.
High-risk flexible endoscopes, such as duodenoscopes and bronchoscopes, have been associated with multiple infectious outbreaks, including those whose causative agents are multi-drug resistant microorganisms. Another reason endoscopes may be considered high-risk is if they’ve been proven difficult to clean. Given the high risk of infection transmission posed by these endoscopes, sterilization is recommended as the best reprocessing choice.
Standards and Guidelines for Sterilizing Endoscopes
Standards organizations such as AORN and ANSI/AAMI are regularly reassessing proper endoscope reprocessing guidelines, and some are now recommending sterilization of endoscopes such as in the ANSI/AAMI ST91 standard.
The ANSI/AAMI Standards Committee shared, "To provide a higher level of assurance, evidence supports the sterilization of all flexible endoscopes, including those used in both semi-critical and critical procedures."6

How to Sterilize Flexible Endoscopes
There are three sterilization methods cleared for use with heat-sensitive, flexible endoscopes:
Ethylene Oxide (EtO) Sterilization uses ethylene oxide gas to eliminate or inactivate microorganisms. Regulations on ethylene oxide use and long cycle times of 24 hours make EtO an impractical option for most facilities.
Vaporized Hydrogen Peroxide (VH2O2) uses hydrogen peroxide vapor to disrupt cell components and eliminate microbes. VHP is compatible with many materials at typical concentrations but may have limitations at higher concentrations. Flexible scopes such as surgical endoscopes and bronchoscopes however can typically be terminally sterilized in VH2O2 sterilizers such as V-PRO Low Temperature Sterilization Systems. Facilities should verify compatibility with the endoscope manufacturer before choosing this option.

Liquid Chemical Sterilization (LCS) of flexible endoscopes offers a high degree of material compatibility using a near-neutral pH solution. Peracetic acid-based systems like the enspire™ 3000 Series Cleaning and Liquid Chemical Sterilant Processing System, or the SYSTEM 1™ endo Liquid Chemical Sterilant Processing System use an immersion process to sterilize intricate devices, including heat-sensitive flexible endoscopes.
LCS of flexible endoscopes achieves fluid penetration into complex lumens to help eliminate all microbial life. Compared to other methods, peracetic acid-based systems enable the shortest cycles, ranging from 18-23 minutes, while providing liquid chemical sterilization of the most complex models, including validated duodenoscopes and gastroscopes.
Storing Sterilized Flexible Endoscopes

After sterilization, an endoscope is removed from the system and typically transferred to storage and drying cabinets. Improperly stored endoscopes can result in contamination and require reprocessing.
Terminally sterilized flexible endoscopes are stored within the tray and packaging in which they were sterilized. Liquid chemically sterilized endoscopes can be used immediately even if wet, or according to ANSI/AMMI ST91, can be stored in a manner similar to HLD5.
Storage and drying cabinets such as the Reliance™ Endoscope Drying and Storage Cabinets or the ENDODRY™ Drying and Storage System provide controlled storage continuous airflow and help prevent cross-contamination of endoscopes.
Transporting Sterilized Endoscopes
When an endoscope is needed for a procedure, they are then transferred to the procedure room using a transport cart, such as the CLEANASCOPE™ ADVANTAGE system. Proper transport of endoscopes will help minimize handling while maintaining separation between contaminated and processed endoscopes.
The Future of Flexible Endoscope Reprocessing
While HLD remains the current standard for flexible endoscopes at many facilities, multidrug-resistant bacteria can survive this process and pose a risk to patients. Only sterilization can eliminate all microbial life, and as flexible endoscope usage continues to increase, standards may mandate sterilization of these devices. Facilities must evaluate infrastructure, workflows, technologies, policies, and training to support this fundamental shift. Adopting LCS systems that achieve liquid chemical sterilization can help prevent infections while supporting patient safety.
Explore STERIS Liquid Chemical Sterilization Products for Endoscopes
Related Resources
Article References
2 https://www.grandviewresearch.com/industry-analysis/endoscopy-procedures-estimates-market-report
3Rutala, 1990 and Spaulding, 1970
4 Rutala WA, Weber DJ. ERCP Scopes: What Can We Do to Prevent Infections? Infection Control & Hospital Epidemiology. 2015;36(6):643-648. doi:10.1017/ice.2015.98
5ANSI/AAMI ST91:2021 Flexible and Semi-Rigid Endoscope Processing in Healthcare Facilities, American National Standard 2022
6 https://array.aami.org/content/news/closer-look-st91-2021-endoscope-processing





