Knowledge Center
May 2, 2024
Endoscope Drying and Storage Cabinet Guidelines
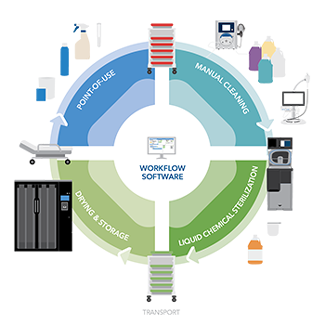
Endoscopes are complex instruments with long, narrow channels that can be difficult to clean and prepare for next procedure. Helping prevent patient infections requires endoscope reprocessing after each use, following a process outlined by the device's instructions for use (IFUs). In addition, many facilities follow recommendations from organizations like ANSI/AAMI, AORN, and SGNA to guide the facilities' approach. Proper endoscope point-of-use treatment, manual cleaning, high level disinfection (HLD) or sterilization, drying and storage are critical to ensure devices are ready for patient use.
This article covers the following topics:
- The importance of endoscope drying and storage
- Standards and guidelines for endoscope drying & storage
- How do you dry an endoscope
- How long it can take to dry an endoscope
- What is an endoscope drying & storage cabinet
- How long can an endoscope be stored (also known as hang time)
- How do you transport an endoscope
The Importance of Proper Endoscope Drying and Storage

While proper storage and drying of endoscopes has received renewed attention from standards organizations in recent years, the critical nature of these practices is not new. More than 40 years ago, Gerding, Peterson, and Vennes published a study in the Gastroenterology journal, noting that forced air drying before storage significantly reduced contamination rates1.
Residual fluid in an endoscope, or any surgical device, can enable biofilm formation, where microorganisms flourish and contribute to possible infection outbreaks5. Thorough drying following reprocessing helps to prevent bacteria growth and potential transmission to patients.
Standards and Guidelines for Endoscope Drying and Storage
Following up-to-date endoscope storage cabinet guidelines and standards is critical for healthcare facilities seeking to optimize reprocessing workflows and ensure patient safety. All major endoscopy (GI) governing bodies and organizations provide standards and guidance for drying and storage.
ANSI/AAMI ST91
The 2021 revision of ANSI/AAMI ST91 provides updated endoscope drying cabinet standards and other considerations, which include:
ANSI/AAMI ST91: 2021
“Methods that employ active drying of endoscopes with filtered air are the preferred means of drying the internal channels of endoscopes after processing.”3
- Actively drying endoscope channels with forced air flow (instrument quality or HEPA-filtered) directed into the lumens using a direct channel attachment for at least 10 minutes.
- Storing in drying cabinets that continually circulate air through endoscopes
- Avoiding the use of syringes for channel drying
- Endoscope-drying storage cabinets are preferable to conventional storage-only cabinets.
- Conducting risk assessments to determine the maximum safe storage duration
AORN
The Association of Perioperative Registered Nurses (AORN) 2022 guidelines align with ANSI/AAMI ST91. They advise drying channels for at least 10 minutes with instrument air or HEPA-filtered air. AORN also guides transport and storage protocols4.
SGNA
The Society of Gastroenterology Nurses and Associates (SGNA) 2018 standards recommend active drying of all endoscope channels and storage in an enclosed, clean cabinet. SGNA advises facilities to determine maximum storage times via risk assessment5.
EN16442
A European standard followed by many countries outside the U.S., EN 16442 specifies performance requirements for storage cabinets designed to store and, if necessary, dry endoscopes following reprocessing6.
How Do You Dry an Endoscope?
Properly drying endoscopes is essential to preventing bacterial growth from residual moisture. Manual and automated drying are the two most common options.
Manual Drying
Manual endoscope drying involves meticulously wiping exterior surfaces and flushing filtered air through each internal channel. Annex K in ST91 provides guidance for technicians to use forced air flow, rather than syringes, for channel drying3.
Automated Drying
Unlike labor-intensive manual methods, automated drying actively facilitates moisture removal from hard-to-reach internal crevices by sustained air circulation. A study in the American Journal of Infection Control reported that automated endoscope drying cabinets shortened external surface drying from 24 hours to three hours and internal channel drying from more than 24 hours to just one hour7.
Alcohol Flushing

While some guidelines, including SGNA, recommend it, other research indicates that alcohol flushing does not provide any drying benefits and can even introduce new reprocessing challenges5.
Alcohol fixation, for example, can exacerbate soil adhesion to surfaces, making thorough cleaning more difficult8. Concentrated alcohol solutions also pose health hazards to staff members9. Furthermore, a study published in the American Journal of Infection Control found that alcohol increased the drying time in some cases11.
Research by STERIS Senior Scientist Michelle Nerandzic aimed to determine the efficacy of alcohol flushing for drying endoscopes and preventing microbial growth during storage10.
The study compared 70%-30% alcohol-flushed channels to those flushed with water alone. Results showed that all alcohol concentrations prevented bacterial growth, but surprisingly, 70% alcohol significantly increased drying time. The study also found that forced air drying effectively prevented bacterial growth, even without alcohol. Flushing with lower alcohol concentrations could aid in microbe control and provide faster drying.
Read the full article here: https://pubmed.ncbi.nlm.nih.gov/36130627/
How Long Does It Take to Dry an Endoscope?

No matter the drying method used, establishing sufficient drying time is challenging since most facilities lack methods to verify channel dryness. Although some guidelines suggest a drying time of at least 10 minutes, the actual process often extends from 90 minutes to several hours, varying based on the complexity of the device.12, 13
Because it's not known when an endoscope is completely dry, using a continuous airflow solution provides the greatest assurance of minimizing residual moisture. Endoscope drying and storage cabinets, such as the ENDODRY™ Drying and Storage System and the Reliance™ 6500 Endoscope Drying and Storage Cabinets, provide this continuous airflow around the devices and even through endoscope channels.
What is an Endoscope Drying and Storage Cabinet?
Endoscope storage cabinets come in vertical or horizontal configurations, which have advantages depending on workflow needs. Unlike a standard storage cabinet, they are specifically designed to store, track, secure, and facilitate drying flexible endoscopes.
Vertical Endoscope Storage Cabinets Benefits
- Sliding panels with removable pegs that allow easy access to endoscopes and flexible storage for different types
- Dedicated drip tray for easy cleaning
- Visibility and access to multiple endoscopes at once
- Designed for high-volume facilities


Horizontal Endoscope Storage Cabinets Benefits
- Endoscopes are secure from swinging and contact with other surfaces that could lead to damage.
- Smaller overall height contributes to space savings
- Smaller footprint to save valuable floor space
Types of Air Filtration in Endoscope Cabinets
Regardless of endoscope cabinet orientation, proper air filtration is required to maintain a clean environment and protect endoscopes during storage. Facilities have two options when selecting systems:
- Instrument Grade Air represents a higher filtration standard, meeting strict criteria (ANSI /ISA–7.0.0–1996) to ensure optimal air quality within the cabinet environment15.
- HEPA-filtered Air is designed to remove 99.97% of particulates from the air, contributing to a clean storage environment16.
Endoscope Drying Storage Cabinet Comparison
 ENDODRY™ Endoscope Drying ENDODRY™ Endoscope Drying
& Storage Cabinet |
 RELIANCE™ 6500 Endoscope Drying RELIANCE™ 6500 Endoscope Drying
& Storage Cabinet |
|
|---|---|---|
| Storage Format |
|
|
| Air Type | A continuous flow of instrument grade air | A continuous flow of HEPA-Filtered Air that removes 99.7% of particulates |
| Capacity Options | Eight endoscopes | 12 or 20 endoscopes, with add-on cabinets available of 12 or 20 more. |
How Long Can an Endoscope Be Stored? (Hang Time)

After drying, endoscopes should be promptly and securely stored to prevent recontamination. The amount of time a disinfected/sterilized endoscope can safely remain in storage, often called "hang time," remains an open debate lacking consensus.
Professional guidance varies widely - from 7 days to 12 weeks - based on reprocessing and storage protocol stringency14. With unclear authority directives, facilities must conduct internal risk assessments, weighing factors like device complexity, drying effectiveness, and endoscope storage cabinet type to develop a policy on hang time.
How do you Transport an Endoscope?

After proper drying and storage procedures, facilities should also consider using dedicated endoscope transport containers.
The Society of Gastroenterology Nurses and Associates (SGNA) states, "Transport the soiled endoscope to the reprocessing area in a closed, puncture-resistant container that prevents exposing staff, patients, or the environment to potentially infectious organisms…"
Endoscope transport carts, such as the CLEANASCOPE™ ADVANTAGE system, minimize handling and maintain separation between contaminated and disinfected endoscopes while providing secure, protective storage to and from the procedure room.
Endoscope Drying and Reliable Storage
Amid mounting concerns over endoscope reprocessing efficacy, organizations now advocate for more stringent and standardized protocols for drying, storing, and transporting endoscopes to safeguard patient well-being. By investing in quality equipment and staff training on guidelines-based processes, healthcare facilities can optimize endoscope workflows, protecting patients from infections.
Explore STERIS Endoscope Drying & Storage Cabinets Here
Related Resources
Article References
1 https://www.sciencedirect.com/science/article/abs/pii/S0016508582801972
4 https://www.aornguidelines.org/guidelines/content?sectionid=173735349&view=book
7 https://www.ajicjournal.org/article/S0196-6553(19)30104-X/addons
8 https://pubmed.ncbi.nlm.nih.gov/28602275/
10 https://pubmed.ncbi.nlm.nih.gov/36130627/
11 https://www.ajicjournal.org/article/S0196-6553(20)30835-X/fulltext
12 https://array.aami.org/content/news/closer-look-st91-2021-endoscope-processing
14 https://array.aami.org/doi/full/10.2345/0899-8205-54.3.223
15 https://www.isa.org/products/isa-7-0-01-1996-quality-standard-for-instrument-ai