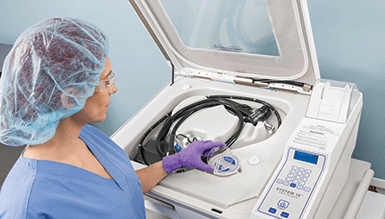
Automated Endoscope Reprocessors
An automated endoscope reprocessor (AER) is the preferred method over manual soaking for reprocessing complex endoscopes. After cleaning, the automated process in an AER circulates either the high-level disinfectant or liquid chemical sterilization solution around the exterior and through the device channels for a timed exposure period, followed by rinsing. STERIS AERs are available in one-sided or pass-thru configurations. Both support best practices by allowing for unidirectional workflow.
Liquid Chemical Sterilization
Liquid chemical sterilization (LCS) provides a higher level of reprocessing assurance for heat-sensitive critical and semi-critical medical devices compared to high-level disinfection. LCS eliminates all viable microbial life, including bacterial spores such as C-Diff, supporting infection prevention best practices. STERIS's automated liquid chemical sterilant processing systems are preferred for low temperature LCS of heat-sensitive complex endoscopes, including flexible multi-channel devices and other high-risk endoscopes such as bronchoscopes, duodenoscopes, cystoscopes, and endobronchial ultrasounds.
*Does not apply to duodenoscopes or endoscopes with an elevator guide wire lever
High-Level Disinfection
High-level disinfection (HLD) kills or inactivates microorganisms that could cause infection when used according to product labeling. Automated processing provides efficient, consistent, and measurable outcomes to eliminate all microorganisms in or on an instrument, except for small numbers of bacterial spores. STERIS helps you maintain a safe and compliant HLD process for your staff and patients with validated products for complex endoscopes.
*Does not apply to Duodenoscopes