Knowledge Center
June 4, 2024
Guide to Reprocessing Robotic Surgery Instruments

Robotic surgery, also called robot-assisted surgery (RAS), is performed using small devices attached to a larger robotic arm. These delicate and complex surgical instruments allow for smaller incisions, better vision, and magnification for the surgeon. The benefits to the patient are faster recovery time and fewer risks of complications.
The first robot-assisted procedure, a brain biopsy, was performed in 1985 using the Programmable Universal Manipulation Arm (PUMA) 560 robot. Since then, the most widely used RAS system is the da Vinci® Surgical System, which has more than 7,500 global installations across 70 countries and more than 12 million procedures performed worldwide1.
Given the benefits of RAS, it's no surprise that these revolutionary technologies continue to experience growth. The robotic surgery market is projected to continue to grow by 13.7% each year until 20302. And it just may be the tip of the iceberg, with 78% of U.S. surgeons expressing a strong interest in adopting surgical robotics technology3.

These numbers will only grow as Medtronic's HUGO™ RAS Systems and other systems from CMR Surgical® and Stryker® continue driving RAS advancement globally. While most RAS systems are used in large hospitals today, they will likely make their way into smaller ambulatory surgery centers (ASCs) in the future.
The Crucial Role of Reprocessing

The instruments used during robotic surgery are inherently complex, with small moving parts where bioburden or debris can remain after a procedure. Proper reprocessing is essential to ensuring devices are ready for use and pathogen-free. Although unique, these complex medical devices do not have specific standards developed for reprocessing and follow the same AAMI & ISO requirements for reprocessing as any other critical device used in surgery.
RAS instruments also represent a significant financial investment for a healthcare facility. A full robotic suite carries an initial investment of $1.5 - 2 million USD plus $100,000-170,000 in yearly service fees. Single-use accessories can cost $700-$3,200 per procedure, highlighting the importance of properly reprocessing the reusable surgical instruments while ensuring they are not damaged.
As RAS growth continues to accelerate, hospitals are challenged to expand capacities and establish surgical procedures to successfully meet the reprocessing needs of these complex and often expensive instruments.
Challenges in Robotic Instrument Reprocessing
Many sterile processing departments (SPDs) face challenges when first adopting a robotic surgery program or as robotic surgery case volume increases. Manual cleaning often requires up to 14 individual steps to clean each device properly. This time-consuming process is highly dependent on SPD technicians completing the steps correctly for each instrument.
Understanding common pain points allows device processing managers to implement effective and efficient policies and procedures to ensure reliable RAS instrument reprocessing. Common pain points for reprocessing robotic instruments include:
- Point-of-use treatment
- Inadequate sink capacity
- Manual cleaning process
- Visual inspection requiring 4x magnification
- Packaging & transporting the delicate surgical instruments
- Ensuring devices are not reprocessed after maximum use
Point-of-Use Treatment of Robotic Instruments
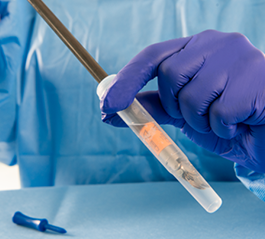
Effective reprocessing requires coordination across the entire periOperative workflow. Without robust point-of-use protocols, bioburden may dry and harden before instruments reach the SPD. Cleaning should start post-procedure at the bedside with point-of-use treatment products specifically designed to keep bioburden moist as instruments are transported back to the SPD. Products like PRE-KLENZ™ Soak Shield are applied after a procedure to keep soils moist, preventing them from adhering to instruments and initiating cleaning by breaking down and removing soils. The plastic vial also helps protect the instrument tips during transport to decontamination and aids in promoting end-user safety since the instrument tips are contained.
Reprocessing Sinks for Robotic Instruments

Priming and soaking robotic instruments are typically required before any cleaning process. Shallow and narrow basins in existing SPD sinks can prevent proper full-arm submersion, which is critical for loosening bioburden post-procedure. Partial soak immersion leads to inadequate exposure of intricate internal mechanisms to cleaning chemistry.
Most instructions for use (IFUs) require RAS arms to occupy sinks for a 30-minute enzymatic soak, tying up sink capacity, delaying additional device cleaning, and causing bottlenecks.
Investing in a dedicated sink like the AMSCO™ 50 Reprocessing Sink with 30-inch bays can support manufacturer instructions without slowing down workflows in decontamination. Coupled with advanced manual cleaning chemistries, like Prolystica® HP Instrument Cleaning Chemistries, to remove surgical soils faster and more completely, wider basins and effective chemistries help optimize workflow.
Manual Cleaning of Robotic Instruments
Cleaning of robotic instruments, like any surgical device, is essential to allow proper sterilization later in the reprocessing cycle. The removal of complex soil contaminants such as cauterized tissues, vaporized smoke plumes, biofluids, and bone dust combine to challenge manual cleaning efficacy.
A missed step could risk leaving residue or debris behind, making manual cleaning one of the possible pain points for sterile processing. Proper cleaning of a typical robotic instrument includes more than a dozen manual cleaning steps per device; therefore, staff training, time, and diligence are important to support successful manual processing.
Through this manual cleaning process, using an ultrasonic cleaner is also necessary, adding to the overall processing time.
Automated Cleaning of Robotic Instruments

Automated cleaning of robotic instruments becomes a valuable alternative. Healthcare facilities can now invest in advanced washers/disinfectors like the AMSCO™ 7053HP Washer/Disinfector that provides a validated cycle for the automated cleaning of select da Vinci® EndoWrist instruments*.
With the RAS (Robotic Assisted Surgery) Cycle, RAS 12 Racks, and Prolystica® Ultra Concentrate HP Enzymatic and Neutral Chemistries, automated cleaning can:
- Drive consistent outcomes using a validated process
- Improve department productivity with up to a 25% reduction in manual cleaning time per set of four instruments4
- Save overall direct labor time of 66 minutes per set of four instruments4
This reduction in hands-on labor also minimizes variability and human error. Investing in systems built for robotics provides the documented, repeatable protocols SPDs need to uphold stringent quality standards.
Inspection of Robotic Instruments
After cleaning, verifying soil removal and assessing damage requires visual inspection. The intricacy of RAS instruments makes this uniquely challenging. Tiny crevices readily conceal microscopic residues, while small defects evade detection without proper magnification. Most robotic instrument IFUs require visual inspection under 4x magnification.
Qualitative Protein Detection tests can be completed to assist with inspection. Some tests allow you to test medical device surfaces, including crevices and ports, for the presence of protein. Whether it's testing the exterior or lumen of a device, you can confirm if residual protein soils are present after the cleaning process, prior to high-level disinfection or sterilization.
Packaging & Transport of Robotic Instruments
Often, RAS instruments are contained in trays of up to four arms and, therefore, delivered to the Operating Room (OR) as a set of four instruments. When the tray is opened, all arms must be reprocessed - even if only 1 or 2 are used during the surgical procedure. Most robotic instruments have a maximum number of uses, so unnecessary reprocessing of instruments that were not used cost both time and use lives of the instrument. Instead of using specialized trays, users can individually pouch and process non-endoscope instrument arms – requiring only instruments used during the procedure to be reprocessed. Be sure to check your specific robotic device Instructions for Use to ensure you are using a validated packaging method.
Tracking Robotic Arm Use
Unlike traditional stainless-steel instruments that withstand hundreds of uses, robotic surgery instruments have strict maximum use cycles due to their complex mechanisms. For patient protection, arms must undergo refurbishment before reaching overuse per manufacturer specifications. Tracking each RAS procedure and arm use is imperative. Instrument tracking systems like SPM™ Surgical Asset Tracking Software enable real-time oversight by automatically logging cycles across each RAS arm's lifespan relative to IFUs.
Planning for Future Robotic Reprocessing Needs
As surgical teams increasingly adopt advanced RAS platforms, dedicated reprocessing technologies and workflows for robotic devices will grow in importance. Reprocessing considerations begin in the OR with point-of-use protocols to enable efficient downstream decontamination and sterilization.
STERIS offers a collection of products designed for RAS instruments' unique reprocessing needs. With double-digit robotic procedure growth annually5, investing in technologies designed for robotic instrument pre-treatment, automated cleaning, inspection, and transport will help prepare SPDs for increased volumes.
Related Resources
Article References
1 Intuitive Surgical, Inc. 2022 ESG Report – Corporate Report
3 https://www.bain.com/insights/navigating-the-next-wave-of-surgical-robotics/
4 STERIS Data on File
5 https://www.strategicmarketresearch.com/blogs/robotic-surgery-statistics
*da Vinci is a registered trademark of Intuitive Surgical, Inc.
The RAS Racks are used in the RAS Cycle of the AMSCO 7052HP Single-Chamber Washer/Disinfector and the AMSCO 7053HP Single-Chamber Washer/Disinfector for the effective cleaning, rinsing, intermediate level disinfection and drying of reusable da Vinci® X/Xi and S/Si EndoWrist® instruments. da Vinci®, EndoWrist® are registered trademarks of Intuitive Surgical.




