Knowledge Center
October 7, 2022

Achieve 510(k) Clearance for Automated Cleaning Process for Select da Vinci Instruments
In 2018, FDA provided guidance around reprocessing instructions for reusable medical devices, which includes robotic surgical instruments. This FDA guidance outlined requirements for manufacturers of Washer/Disinfectors to validate reprocessing instructions, specifically when indicating them for use with reusable medical devices such as with da Vinci EndoWrist® instruments.1 The updated guidance limited reprocessing to a manual cleaning process in the US. In addition, device manufacturers must demonstrate thermal disinfection for Washer/Disinfectors used to disinfect da Vinci instrumentation.1
Per FDA guidance, a medical device with a specific maximum number of reprocessing cycles should not have it's maximum number exceeded. Sterile Processing Departments (SPD) must track reprocessing cycles per Intuitive Reprocessing Instructions.1 Each da Vinci EndoWrist S/Si and X/Xi instrument has a specific number of uses and reprocessing cycles. Through continuous innovation, Intuitive provides a buffer in reprocessing EndoWrist S/Si and X/Xi instruments; 5 additional reprocessing cycles per instrument.2
- Based on the new guidance, Intuitive and STERIS collaborated to achieve a 510(k) cleared automated cleaning process with thermal disinfection for da Vinci EndoWrist S/Si and X/Xi instruments.3
- Through the collaboration, STERIS and Intuitive successfully validated the automated cleaning process for da Vinci EndoWrist S/Si and X/Xi instruments in the AMSCO® 7052HP and 7053HP Washer Disinfectors using the RAS Cycle, RAS 12 Racks, and Prolystica® Ultra Concentrate HP Enzymatic and Neutral Chemistries.4
- Both parties also validated cleaning efficacy in accordance with AAMI TIR 30:2011/(R)2016.5
- STERIS and Intuitive worked together to help simplify the automated cleaning process, creating easy-to-follow instructions that help create cleaning outcomes consistent with the manual cleaning process.
This automated cleaning system may provide enhanced SPD operation efficiency,7 a less labor intensive cleaning process,7 consistent cleaning efficacy,8 and more reprocessing choices to our Customers – which is the continuous innovation that Intuitive and STERIS strive to achieve.
Regulatory Path
Each company sought 510(k) clearance, STERIS for the RAS 12 Racks and Cycle, and Intuitive for the cleaning solution and updated instructions for use. A collaborative effort helped support clearance for both companies to accomplish each submission to the FDA, which was a unique strategy in that washers/disinfectors are 510(k) exempt, while the RAS Racks and Cycles require 510(k) clearance.
The collaboration involved not only sharing of validation protocols and devices but also included a review of each company’s Instructions for Use to make sure mutual Customers would be able to pre-clean and reprocess da Vinci Endowrist instruments in the AMSCO 7000 Series Single-Chamber Washer-Disinfector.
Establishing Worst Case Conditions

To validate the efficacy of the automated cleaning process, it was first necessary to quantify the amount of soil that enters the instrument shaft. Since this area cannot be visually inspected by the user, it was important to create shaft soil loads for cleaning validation that was representative of soil loads encountered during typical use. Factors involved in doing this included clinical case review and choosing a “worst case” soiled load based on typical instrument choreography. A simulated procedure was performed using a live porcine model. Following the procedure, all soil that entered the instrument shaft was extracted and analyzed for total protein content. This established the standard for worst case soil loads.

After establishing worst case soil loads, worst case instrument categories were identified. Monopolar and bipolar cautery instruments were identified as the most challenging instruments to clean due to the presence of charred tissue and soil present following a surgical procedure. A simulated surgical procedure included exposure to extensive charring to represent worst case soiling during typical use. This simulated procedure also sought to mimic the real-life challenge of cleaning devices with dried soil.
Cleaning Validation
During cleaning validation, instruments were subjected to repeated cycles of simulated surgical use, shaft soiling, manual pre-cleaning, automated cleaning and thermal disinfection, and steam sterilization to capture the potential of instruments to accumulate soil over their expected life. Worst case cleaning considerations were also included for point of use and variability of manual pre-cleaning steps.

Validation of cleaning efficacy included analytical assays, as well as magnified visual inspection of disassembled instruments. Extraction techniques ensured that all residual protein and hemoglobin were captured. Protein and hemoglobin values were assessed against industry accepted criteria. The efficacy of the cleaning process was determined by showing that protein and hemoglobin values met the industry accepted criteria and no residual soil was visible on the disassembled instruments.
Clinical Verification
Devices used in robotic assisted surgery have small, complex working features that require a robust cleaning process, presenting multiple challenges to the sterile processing department. It was important to test the automated cleaning process in a clinical application.
Three sets of most commonly utilized instruments were transported to testing facilities after patient use with no point of use steps. The instruments were processed within 120 minutes after the procedure was completed. The cleaned instruments were sampled to test for protein, total organic carbon (TOC), and instruments were visually inspected under magnification. The residual values for both protein and TOC were well below industry accepted levels.
This demonstrated the effectiveness of the RAS 12 Rack and the RAS Cycle in the AMSCO 7052HP SingleChamber Washer/Disinfector with Prolystica Ultra Concentrate HP Enzymatic Cleaner and Prolystica Ultra Concentrate HP Neutral Detergent to achieve cleaning across a variety of da Vinci EndoWrist instruments used in clinical procedures.
Reprocessing Efficiency
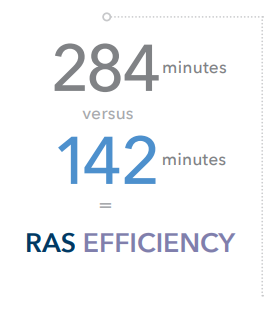
Decontaminating da Vinci Endowrist instruments using a manual process presents a challenge to a hospital’s sterile processing department due to the time-consuming steps. Variations in operating room case volume could further strain sterile processing resources. The manual decontamination process for da Vinci Endowrist instruments includes 15 steps, taking up to 284 minutes for 12 Endowrist instruments.9 12 instruments represents up to 3 surgical procedures performed using da Vinci surgical systems. See the table below for detailed steps for the cleaning process.
Several steps are eliminated in the automated process, including flushing the instruments and the use of an ultrasonic cleaner.9 These steps can be eliminated by introducing a mechanized cleaning action in the AMSCO 7052HP and 7053HP Washer Disinfectors and RAS Racks and Cycle. This mechanical action is achieved using high pressure impingement with pressurized water and chemistry on the instruments’ exterior and pressurized water and chemistry through the da Vinci Endowrist channels to provide contact in all areas of the instrument. Using the RAS Cycle and RAS Rack in the AMSCO 7052HP or 7053HP Washer Disinfector, the automated cleaning process for 12 Endowrist instruments involves 10 steps and takes 142 minutes, including the washer cycle time.
By eliminating several manual steps, sterile process staff can focus on other important tasks in the department such as assembling instrument trays for the sterilization process. In addition, the ultrasonic cleaner can be utilized for reprocessing other devices that require ultrasonic cleaning such as orthopedic surgery instruments. Overall, the automated process allows for more efficient use of staff and equipment resources in the sterile processing department.
| Manual Decontamination Process | ||
|---|---|---|
| *Average processing times include sink filling and draining sequence, transfer time between steps and complete sonic cycle for 12 instruments | ||
| Step 1 | Remove Accessories | |
| Step 2 | Check Indicator | |
| Step 3 | Prepare Solution | |
| Step 4 | Prime | |
| Step 5 | Soak 30 Minutes | |
| Step 6 | Flush | |
| Step 7 | Spray | |
| Step 8 | Brush | |
| Step 9 | Rinse | |
| Step 10 | Ultrasonic Cleaning | |
| Step 11 | Flush | |
| Step 12 | Final Rinse | |
| Step 13 | Final Decon Inspection | |
| Step 14 | Thermal Disinfection | |
| Step 15 | Transfer | |
| Average Time* (12 instruments) |
284 Minutes | |
| Automated Decontamination Process Using RAS Cycle | ||
|---|---|---|
| *Average processing times include sink filling and draining sequence, transfer time between steps and complete sonic cycle for 12 instruments | ||
| Step 1 | Remove Accessories | |
| Step 2 | Check Indicator | |
| Step 3 | Prepare Solution | |
| Step 4 | Prime | |
| Step 5 | Soak 10 minutes | |
| Step 6 | Spray | |
| Step 7 | Brush | |
| Step 8 | Inspect | |
| Step 9 | Automated Cleaning and Disinfection | |
| Step 10 | Transfer | |
| Average Time* (12 instruments) |
142 Minutes | |
Conclusion
STERIS and Intuitive collaborated to develop, optimize, and validate an automated cleaning process for da Vinci instruments. Cleaning validations were conducted to reproduce worst case soiling conditions and simulated surgical maneuvers. Cleaning parameters in the IFU were minimized in order to mimic the worst-case cleaning scenario that may be used in a hospital. This joint effort resulted in a robust automated cleaning process that can deliver clean instruments while minimizing manual reprocessing steps.
Contributors
Derek Mortisen, PhD, Intuitive
Veronica Thralls, PMP, Intuitive
Sarah Brown, MBA, STERIS
Herbert J. Kaiser, PhD, STERIS
Amy Thanavaro, PhD, STERIS
Nupur Jain, Intuitive
Article References
1 FDA Guidance for Reprocessing Medical Devices in Health Care Settings: Validation Methods and Labeling, Guidance for Industry and Food and Drug Administration Staff Document Issued on March 17, 2015.
2 For validated number of reprocessing cycles for all instruments, refer to Intuitive reprocessing appendices 552268-03 and 552246-07.
3 K190081, K200577, K203199, K203632.
4 For validated instruments, refer to Intuitive reprocessing appendices 552268-03 and 552246-06.
5 A compendium of processes, materials, test methods, and acceptance criteria for cleaning reusable medical devices, AAMI TIR 12:2010, Designing, testing, and labeling reusable medical devices for reprocessing in health care facilities: A guide for medical device manufacturers, and FDA Guidance for Reprocessing Medical Devices in Health Care Settings: Validation Methods and Labeling, Guidance for Industry and Food and Drug Administration Staff Document Issued on March 17, 2015.
6 Internal data on file
7 Compared to manual cleaning process.
8 Similar level of cleaning efficacy compared to manual cleaning process. Internal data on file.
9 Refer to reprocessing manuals for manual and automated cleaning steps


