







AMSCO® 400 Series Small Steam Sterilizer
- Ideal for quick turnaround in the Operating Room or Sterile Processing Department
- Validated "immediate-use" steam sterilizer with two chamber sizes available
- Configure and name cycles to specific facility needs
- Eliminate the need for Bowie-Dick testing with an Optional SFPP cycle
Product Overview
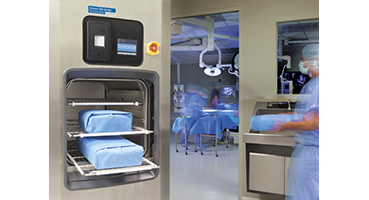
The AMSCO 400 Series Small Steam Sterilizer (autoclave) provides efficient sterilization for space constrained facilities. Engineered for staff and patient safety, while meeting AAMI/AORN guidelines, the 20" x 20" small autoclave is validated to accommodate up to three 25-lb sets.
How Does the AMSCO 400 Small Steam Sterilizer Work?
These autoclaves are designed for fast and efficient steam sterilization of heat- and moisture-stable materials. The AMSCO 400 Series Small Steam Sterilizer is equipped with prevacuum, gravity, liquid, and all applicable test cycles. An optional Steam Flush Pressure Pulse (SFPP) configuration adds this cycle to your autoclave, eliminating the need for Bowie-Dick testing which reduces operating costs.
Why Choose the AMSCO 400 Small Steam Sterilizer?
The AMSCO 400 Series Sterilizers are designed for quick turnaround in the OR and SPD, while meeting AAMI/AORN guidelines and reducing water usage per pound of instruments processed.
Each steam sterilizer is equipped with either a single door for open or recessed mounting, or double door configuration for optimal workflow. Additional features include:
- Vertical sliding door with foot pedal for hands-free loading and unloading
- Industry's only 2 Year warranty on the non-lubricated, steam activated gasket
- Available with fully integrated steam generator including standard flush and drain capability
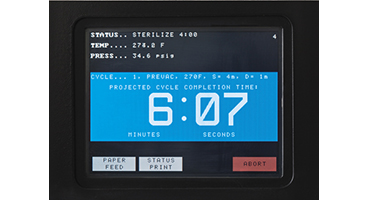
- Touch screen controls provide a user-friendly interface
- Available with optional water-saving electric vacuum pump
Sterilizer Dimensions and Chamber Sizes
| AMSCO 400 STEAM STERILIZER | INTERIOR CHAMBER DIMENSIONS | MAX. OUTSIDE DIMENSIONS |
|---|---|---|
|
16X16 Models |
16” x 16” x 26" (406 x 406 x 660mm) |
36 7/8”*L x 26”w x 74 ½h” |
|
20x20 Models |
20” x 20” x 38" (508 x 508 x 965 mm) |
45 5/8”*L x 30”w x 74 ½h” |
Utility Requirements
For complete autoclave utility requirements please see the Tech Data sheet on the Literature Tab. Hospitals can choose to either use facility steam, or a fully integrated steam generator. Utility requirements for the AMSCO 400 Steam Sterilizer varies based on unit specifications.
*May vary slightly by specific model configuration.
Genuine STERIS Parts for your STERIS Steam Sterilizers
By choosing genuine STERIS OEM replacement parts, you can be assured that each and every part is chosen based on the highest quality standards to ensure your equipment functions at its best. STERIS's OEM replacement parts promote compliance with regulatory requirements and organizational standards on equipment maintenance and repair.
|
OPERATORS MANUAL
|
|
|---|---|
| Document # | Document Title |
| AMSCO 400 SERIES SMALL STEAM STERILIZERS (PT-BR) | |
| AMSCO 400 SERIES SMALL STEAM STERILIZERS (TR) | |
| AMSCO 400 SERIES SMALL STEAM STERILIZERS (FR) | |
| AMSCO 400 SERIES SMALL STEAM STERILIZERS (ES) | |
|
INSTRUCTIONS FOR USE
|
|
|---|---|
| Document # | Document Title |
| AMSCO STEAM STERILIZER ROUTINE CLEANING PROCEDURE – IFU | |
| STEAM STERILIZER LEAK TEST RESULTS LOG | |
|
WALL CHARTS
|
|
|---|---|
| Document # | Document Title |
| STEAM CYCLE TAPE INTERPRETATION WALL CHART | |
| ROUTINE CHECKLIST FOR ALL AMSCO SMALL, MEDIUM AND FLOORLOADER STEAM STERILIZERS | |
| ROUTINE CHECKLIST FOR ALL AMSCO SMALL, MEDIUM AND FLOORLOADER STEAM STERILIZERS | |
| ROUTINE CHECKLIST FOR ALL AMSCO SMALL, MEDIUM AND FLOORLOADER STEAM STERILIZERS | |
|
BROCHURE
|
|
|---|---|
| Document # | Document Title |
| AMSCO STEAM STERILIZER FAMILY BROCHURE | |
| AMSCO 400 SERIES SMALL STEAM STERILIZER BROCHURE (.PDF ONLY) | |
| AMSCO 400 SERIES SMALL STEAM STERILIZER BROCHURE (FRENCH) | |
|
TECHNICAL TIPS
|
|
|---|---|
| Document # | Document Title |
| GUIDE TO OPTIMAL STEAM GENERATION | |
| GUIDE TO STAINLESS STEEL CORROSION REMOVAL | |
| GUIDE TO STEAM STERILIZATION CYCLES - STEAM FLUSH PRESSURE PULSE | |
|
SELL SHEET
|
|
|---|---|
| Document # | Document Title |
| SECURECARE WET PACK SOLUTION TOOL KIT | |
|
TECH DATA SHEET
|
|
|---|---|
| Document # | Document Title |
| AMSCO 400 SMALL | |