Knowledge Center
October 28, 2024
SPD Staffing & Training: Critical to An Effective Sterile Processing Program
What are the roles and responsibilities in the SPD?

The Sterile Processing Department (SPD) is responsible for cleaning, disinfecting, and sterilizing devices and instruments used in surgical procedures. Effective sterilization of surgical instruments and devices is critical to patient safety. Typical roles in the SPD consist of sterile processing technicians, managers, supervisors, and educators. Many SPDs also employ an operating room (OR) liaison to improve communication and collaboration between the OR and SPD. Each person in the SPD performs specific tasks in coordination with one another to ensure instruments are sterilized properly. Improperly processed surgical instruments can impact patients' safety. Ensuring your staff is adequately trained, certified, and understands procedures and policies is vital.
Learn more about what sterile processing is
What is the job description of a sterile processing technician?

The SPD technicians' primary role is to clean, sterilize, store, and track surgical instruments and devices used in medical procedures. The title of a sterile processing technician can be used interchangeably with Central Service Technician (CST), Sterile Processing Department (SPD) Technician, Certified Sterile Science Technician (CSST), Central Sterile Supply (CSS) Technician, and Central Processing Technician (CPT).
Sterile processing technicians work behind the scenes at healthcare facilities and play a huge role in patient safety. Technicians must ensure that all surgical instruments and medical equipment are properly sterilized, assembled, prepped, packed, and stored for reuse. In addition, technicians must be ready to work in a fast-paced, detail-oriented department and comply with the following standards:
- Department of Health (DOH)
- The Joint Commission (TJC)
- Occupational Safety and Health Administration (OSHA)
- Centers for Disease Control and Prevention (CDC)
- Association for the Advancement of Medical Instrumentation (AAMI)
- Association of periOperative Registered Nurses (AORN)
- The healthcare facility's goals and policies
The Role of a Lead Tech in the SPD
The key to a successful SPD operation is ensuring there is leadership on the floor for all shifts. It is recommended that lead technicians be a part of your staffing mix. The roles and responsibilities can be demanding, as the role of an SPD technician can vary based on the facility and the types of surgical procedures a facility specializes in, requiring a strong lead technician on each shift.
Technicians must understand surgical instruments, medical devices, and trays of various complexities to ensure all instruments are properly sterilized and stored based on the Manufacturer’s Instructions for Use (IFU). With the industry being impacted by staffing shortages, various SPD job opportunities exist for individuals looking to work in sterile processing.
The Role of a Manager in the SPD

The sterile processing manager provides leadership oversight and planning. Managers, like technicians, must also have active certifications from governing bodies in certain states and healthcare facilities. They lead training efforts and ensure proper policy implementation. SPD managers must have adequate knowledge of their department to ensure they are managing their employees and processes properly for overall department efficiency.
An SPD manager also acts as a liaison between their department and other departments throughout healthcare facilities by overseeing that instruments are properly sterilized, prepared, stored, and ready to be returned to the Operating Room (OR). Any interruption in the sterilization process could lead to delays in the OR, surgical errors, and risks to patients' safety.
The Role of Supervisors in the SPD
The SPD supervisor oversees and drives productivity. They also assist and support daily operations for the decontamination, reprocessing, and sterilization at a healthcare facility. They provide onsite support, training, and communication for the SPD and ensure they comply with the DOH, TJC, OSHA, CDC, AAMI, and AORN standards, facilities goals, and policies.
This position oversees new hire training and facilitates educational opportunities for the department. The SPD supervisor can lead staff training programs and opportunities to acquire the knowledge and credits required to maintain certifications. In addition, the supervisor aligns with the OR schedule and ensures proper inventories of surgical instruments.
The Role of an Educator in the SPD

A sterile processing educator's role is to provide the SPDs with educational support, including a needs assessment. A needs assessment is the process of evaluating the training and educational needs of individuals, groups, or organizations and aligning their needs with the curriculum.1 The educator position provides standardized education in the area of expertise and general education, including but not limited to orientation, annual competency assessment, hands-on training, regulatory, accreditation, and certification. In addition to educating, they will assess and develop SPD policies and maintain associate certification requirements.
Learn more about our Sterile Processing Education and Training offerings, which provide hands-on, classroom-based, and virtual options for the SPD.
Creating an Effective Sterile Processing Program
Shift Planning in the CSSD
Shift planning in the CSSD is one of the most critical actions that leadership needs to focus on daily. Considerations include surgical procedure volumes, staffing, equipment, and instrument availability, which should all be evaluated to create a production plan for each shift. Shift planning helps leadership move resources within the department to address heavy production demands in decontamination, assembly, and sterilization. Understanding your production standards for each employee and the equipment within the department will help determine where the resources need to be allocated throughout the shift.
SPD Staffing & Hiring
Staffing shortages continue to impact sterile processing. Not only does the lack of labor result in lower productivity for the SPD, but it poses a considerable risk to patient safety and quality. Some healthcare facilities are looking at alternatives, such as an SPD staffing agency, to help fill staffing gaps. Some of these alternatives include:
- Emergency Staffing – Rapid deployment of specialists to help decrease significant instrument tray backlogs at a facility.
- Interim/Temporary Staffing – Staffing service that helps fill a temporary need at a facility. They are traditionally provided based on 13-week terms.
- Long-Term Staffing – Technicians are recruited and hired as local specialists for long-term contracts at a facility.
- Offsite Reprocessing Centers (ORCs) – Instruments are transported offsite to an ORC, where technicians process the instruments and transport them back to a healthcare facility. Utilizing an ORC can help alleviate capacity and staffing constraints associated with surgical case volume increases.
Pros and Cons of Sterile Processing Staffing Agencies
| Pros | Cons |
|---|---|
|
|
Staffing agencies should be reputable, know their candidates, and provide training opportunities to employees with entry-level experience. Some considerations to look into when selecting a staffing agency can be:

- Prescreen their traveler candidates (ensuring background checks and drug screens are completed before arrival).
- Require annual physical examinations to declare traveling technicians fit for duty (including ensuring current immunizations).
- Ensure certifications are up to date.
- Have the ability to provide a replacement traveler within a specified period of a traveler leaving suddenly or being let go.
Communication in the Sterile Processing Department
Communication is critical to providing efficient periOperative services. Various departments must coordinate the services they provide. Any communication breakdown can impact patient safety, increase costs, and delay surgical procedures. As surgical instruments and medical devices become increasingly complex, coordination between the OR and the SPD is critical. The OR must communicate their instrument needs with the SPD to ensure that instruments are sterile and available on time for procedures. In addition, the SPD should communicate with the OR about concerns of instruments being returned to the SPD for reprocessing.
Automation in the SPD
Automation refers to any activity or process performed automatically, usually by machines or software applications. Within SPDs, automation advancements have helped increase productivity and improve quality while the future of Artificial Intelligence (AI) and Augmented Intelligence are being tested to bring further improvements. Historically, the first automation to revolutionize sterile processing was the introduction of automated washing equipment and steam sterilizers by replacing hand washing, boiling water, and bleach sterilization techniques. These automations were replaced with equipment that could repeatedly perform the tasks to quality standards while removing variations, improving the work environment and the patient experience by reducing potential infections.
In today’s SPD, you will find various automated equipment, i.e., cart washers, ultrasonics, and instrument washers. A few hospitals also use Automated Guide Vehicles to transport instrument case carts and supply carts. Computer software has also automated standard processes within SPD, including linking the surgery schedule to the sterile processing inventory and tracking system to indicate which instrument trays are needed for surgery.
Instrument tracking systems have automated the tracking of surgical instruments through barcodes and even RFID (radio frequency identification) for inventory control. Manufacturer’s IFUs that provide technicians with instructions on processing instruments can be delivered automatically through software, ensuring technicians complete each required step. Equipment cycle information and biological incubator results can be automatically linked and downloaded to instrument tracking systems. Visual identification of surgical instruments is currently being pursued through AI applications to assist technicians in identifying instruments. Automation and software applications will continue to drive advancements in healthcare to improve outcomes and efficiencies.
SPD Quality Standards and Productivity Metrics
SPD Quality Standards
Quality standards in the SPD are based on insights and improve outcomes through evidence-based practice, metrics, and benchmarking that allow SPDs to analyze variation in quality measures and identify research opportunities that advance professional knowledge, which informs the creation of future best practices.3 There are many ways to measure quality within sterile processing. It is important to identify what best gauges the overall health of the department's practices. The goal of any SPD is to provide sterile, quality instrumentation for all patients, and a facility’s quality measures should reflect that goal.
One quality standard measured in the SPD is the outcome of sterile surgical instrument trays. Instrument tray Issue/Quality is a quality metric that allows the SPD to analyze variation in tray quality measures, complete a root cause analysis, and develop countermeasures. For example, tray Issue/Quality identifies the variations that compromise the integrity of a sterile, reusable product and measures the frequency of instrumentation provided to the Customer (surgery) found to have a tray quality issue regardless of fault. This metric is calculated in two ways:
- The number of related defects/issues (numerator) divided by the total trays processed (denominator).
- The number of related defects/issues (numerator) divided by the number of cases (denominator). This data is used to detect trends and establish root causes and viable corrective action plans.
This visual is an example of the STERIS Instrument Processing Standard Quality Board used to track and communicate quality measures in the SPD.
Productivity Metrics in the SPD
Most of the time, SPD productivity metrics are based on standard metrics such as the number of surgical instruments that are sterilized or disinfected and the number of labor hours to complete this work. Although this sounds straightforward, there are a lot of various steps within the reprocessing cycle. You must first understand all the required steps to establish your SPD productivity metrics.
- First, you must follow the instruments through the reprocessing cycle, starting with instruments being delivered into decontamination. Observe the required steps. When observing these tasks, count the number of instruments being cleaned and placed on the washer rack, note when your observations start and stop, and count the number of sterile processing technicians working.
- You continue these steps for assembly, sterilization, and case cart building. Once this is complete, use the data collected to establish the baseline for your current state of productivity. For example, 75 minutes observed X 2 technicians /35 instrument sets and devices completed = 4.5 minutes per Tray/Device.
- Continue this process in other areas and tasks within the SPD to determine the labor hours needed to complete all tasks.
- The next step is establishing productivity expectations by calculating hourly productivity numbers. i.e., Based on the formula above, you can assume that one technician can decontaminate a tray/device every 4.5 minutes or 13 per hour.
- Communicate the expectations, methods for measuring, and frequency to ensure your department understands what is being asked of them. Testing your calculations and making any alterations before finalizing and sharing with the team is always important.
Various factors can impact productivity in the SPD, including unclear expectations, lack of accountability, incoming quality issues, disruptions, and lack of supervision. In-depth assessments can identify process improvement options and provide strategic recommendations for your SPD operations and performance. Learn more about our Sterile Processing Consulting and Performance Improvements offering.
SPD Certifications your Sterile Processing Team should hold
SPD Training and Certification

Whether you are advancing in your sterile processing career or just starting, a wide breadth of training options is available. Training courses for SPD Technicians can differ depending on the state of residence. Some states can require more thorough training and certifications to become a technician and work in a healthcare facility. The increase in certifications and skills among sterile processing professionals is backed by the desire to reprocess instruments safely and effectively to minimize patient risk at facilities. There are several training programs to choose from. It is important to understand the requirements in your state of residence and find training that meets all your needs.
To start your sterile processing journey, you will choose and enroll in a training program that best meets your needs. Training programs can be full or part-time, virtual, or in-person. Once all the necessary coursework hours are completed, the next step is to enroll in a clinical internship at a healthcare facility. This allows potential technicians to apply what they have learned in their training programs to real-life situations.
After the clinical internship is complete, the next step is to pass the Certified Registered Central Technician (CRCST) exam or the Certification Board for Sterile Processing and Distribution (CBSPD) sterile processing technician certification. These introduction courses certify that an individual can perform the role of an SPD technician.
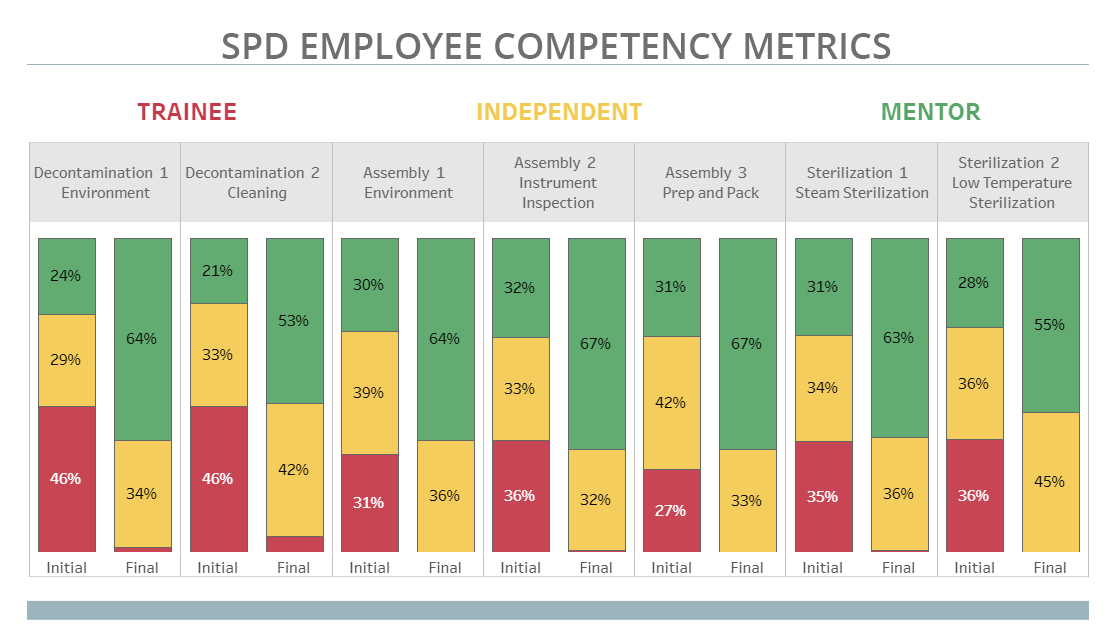
This graph visualizes where employees' competencies are based on their education and training in the SPD.
SPD Continuing Education
As sterile processing becomes more complex, recertification is required every 1 to 5 years, depending on the certification the organization requires. Recertification is achieved through retesting, continued work in the profession, and/or continuing education.2 Continuing education units (CEUs) help SPD technicians, supervisors, educators, and managers refine their skills and stay informed on the latest industry standards. The total CEU hours needed are dependent on the certification organization and the actual certifications.
Healthcare Sterile Processing Association (HSPA) over ONE year are: |
Certification Board for Sterile Processing and Distribution over FIVE years are: |
|---|---|
| Certified Registered Central Service Technician (CRCST) 12 CEU credits | Certified Sterile Processing Technician (CSPDT) 100 CEU credits |
| Certified Instrument Specialist (CIS) 6 CEU credits + ongoing CRCST | Certified Surgical Instrument Specialist (CSIS) 100 CEU credits |
| Certified Endoscope Reprocessor (CER) 6 CEU credits | Certified Flexible Endoscope Reprocessor (CFER) 100 CEU credits |
| Certified Healthcare Leader (CHL) 6 CEU credits + ongoing CRCST | Certified in Sterile Processing Management (CSPM) 100 CEU credits |
| Certified Central Service Vendor Partner (CCSVP) 6 CEU credits | Certified Ambulatory Surgery Technician (CASSPT) 100 CEU credits |
CEUs can be obtained online through webinars, videos, and lesson plans. To stay up-to-date on your CEUs, these free CE courses for healthcare professionals are approved for CE Credit by CBRN, CBSPD, and HSPA, with select courses approved by the American Board of Certification for Gastroenterology Nurses (ABCGN).
The Certified Registered Central Technician Exam (CRCST)
Administered by HSPA, it is designed to recognize entry-level and existing technicians who have demonstrated the experience, knowledge, and skills necessary to provide competent services as a Sterile Processing technician.3 In addition to passing the exam, you must also have 400 hours of hands-on experience within the past five years of passing the exam. Hands-on experience must be completed in an SPD and can be paired or on a volunteer basis. The certification is valid for one year, and to renew the credentials, you must take CEU courses and pay an annual fee.
The Certification Board for Sterile Processing and Distribution (CBSPD)
Formerly known as the National Institute for the Certification of Healthcare Sterile Processing and Distribution Personnel (NICHSPDP), CBSPD offers an entry-level certification for sterile processing technicians to ensure they are knowledgeable enough to perform the role adequately. The certificate is valid for five years, and individuals must recertify through CEU courses.
HSPA or CBSPD: Is there a difference?
Newcomers to sterile processing often need clarification about which certification program they should enroll in, HSPA or CBSPD. Despite what many people think, both certifications are valid throughout the United States. The exams are different, but they contain similar information. Hiring managers are not biased toward one certification or another because they understand that both sterile processing certifications have rigorous requirements. To pass the CRCST or CSPDT exam, you will need a passing score of at least 70%.
ANSI/AAMI ST79 Standard
ANSI/AAMI ST79 is a comprehensive guide to steam sterilization and sterility assurance for any healthcare personnel using steam. This document helps ensure safety and proper sterilization practices for reprocessing surgical instruments. It also acts as a guide to conform with the latest industry standards for TJC and remains the go-to document for their surveys.
STERIS Education & Training Programs
STERIS provides a breadth of educational and certification readiness offerings from new hire education to training and onboarding an educator onsite at your healthcare facility. Our SPD leaders and technicians are trained on HSPA, CBSPD, and ANSI/AAMI ST79 standards, providing you and your team with education customized to the department's needs and objectives. The benefits of STERIS SPD education and training programs include:
- Increased efficiency and productivity
- Enhanced quality control and regulatory compliance
- Improved communication and collaboration within the department
- Team morale
- Confident Associates
- Standardized onboarding and training plan
Contributors

John Kimsey
Vice President of Processing Optimization and Customer Success
John brings 20+ years of experience in the Sterile Processing (SPD) / Central Sterile Services Departments (CSSD). In working with the leading hospital systems worldwide, John has vast expertise in analyzing and advising sterile processing strategy, operational improvements, capacity planning, staffing analysis, and clinical and regulatory assessments for single and multi-location systems.

April Burgess
Senior Clinical Education Manager
April Burgess has 26 years of experience in the operating room and sterile processing field, the last 10 of those specific to sterile processing education and leadership. She is a multi-skilled professional with experience in teaching, training, quality control, assessments and implementing policies and procedures. April joined STERIS in 2015 and has spent the last 4 years supporting the education team across the United States in Sterile Processing Departments at various locations.

Christy Newland
Director of Performance Improvement, Instrument Processing
Christy has over 20 years of experience in various clinical roles within Surgical Services as well as Multi-site Director of Operations SPD, Senior Professional Consultant, Process Improvement Director, and Regional Director of Operations for seven hospitals in Ohio. Christy is skilled in streamlining operations and improving throughput and quality through continuous process improvement, quality assurance, regulations, and cross-functional collaboration.

Kevin Smysor
Director, Field Operations
Kevin brings 31+ years of experience in OR and sterile processing operations with 20+ years in leadership roles. He has focused on developing, implementing and managing specific services offering for Customer nationwide to improve efficiencies within the OR and SPD. Kevin continues to focus on ensuring IP Operational leadership delivers positive outcomes for our Customers nationwide.
Related Resources
Article References
1 Salas E, Cannon-Bowers JA. The science of training: a decade of progress. Annu Rev Psychol. 2001;52:471-99. [PubMed]
2 https://www.cbspd.net/about-ceus/
3 https://myhspa.org/certification/iahcsmm-certifications.html