Knowledge Center
February 1, 2021
Guide to Gravity IUSS Cycle Monitoring
Routine monitoring of steam sterilizers with biological indicators (BIs) applies to all types of cycles performed in autoclaves – including immediate use steam sterilization (IUSS) cycles. Previously referred to as "flash sterilization," these cycles should only be used in emergencies when no other options are available.
This article reviews the importance of sterility assurance monitoring, the best practice standards for sterility assurance monitoring, and how to establish a successful monitoring process for gravity IUSS cycles.
When can I use immediate use steam sterilization cycles?
AAMI Standards and AORN Guidelines agree that Immediate Use Steam Sterilization should be kept to a minimum and should only be used in situations where no other option is available.
There may be a few situations where a facility may determine that running an IUSS cycle in an autoclave is the only option, if so, the facility should define what constitutes an emergency in their policy and procedures. Staff should be trained as to what constitutes an emergency, and some examples include:
- When a specific instrument is needed for an emergency procedure and is not sterile
- When a non-replaceable instrument has been contaminated and is needed for a procedure that cannot be rescheduled
- When an item falls on the floor and is needed to continue an in-process surgical procedure
What are the different types of cycles available for IUSS?
IUSS cycles are not standard, when situations arise the user must understand the differences between the types of IUSS cycles and how to properly monitor them. Three different types of Immediate-Use Steam Sterilization cycles may be available on your specific steam sterilizers: gravity, prevacuum, and express.
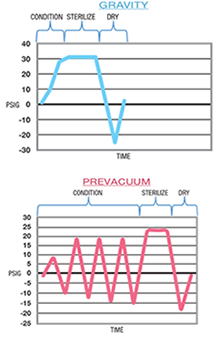
All immediate use steam sterilization cycles have three phases:
- Conditioning phase: Air is removed from the chamber and load contents. The cycle load is then brought up to the programmed sterilization temperature.
- Sterilization phase: The chamber remains at the programmed temperature for the programmed amount of time.
- Exhaust phase: The steam is removed from the chamber, but dry time is insufficient to dry the load so the container and instruments may be moist.
Can I use the Express IUSS Cycle?
AAMI ST79:2017 defines an express cycle as "Immediate Use Steam Sterilization (IUSS) with a single wrapper." Overall, the express cycle should not be used because the wrapper does not provide adequate protection against device contamination during transport from the sterilizer to the point of use. This article focuses on gravity and prevacuum IUSS cycles.
When can I use Gravity IUSS cycles?
Gravity steam sterilization cycles use gravity in the conditioning and exhaust phases to displace air and steam, which is known as a passive process. At one time this was the only available cycle, so there may be some steam sterilizers that still only have gravity capability, but typically these sterilizers are past their recommended life cycle. Gravity IUSS sterilization cycles are also used when the device manufacturer's written Instructions for Use (IFU) requires a gravity steam sterilization cycle for the device. It is critical to make sure the medical device manufacturer's written IFU, sterilizer and container is validated for the specific cycle that is selected.
To monitor these cycles, you must use gravity validated sterility assurance products that test the sterilizer's ability to kill spores. Both non-porous and porous items can be sterilized for immediate use if the appropriate time and temperature are used. Below are examples of common gravity IUSS sterilization cycle parameters. Actual sterilization times and temperatures are determined by medical device manufacturers and will be listed in the written IFUs.
| DEVICE TYPES | IUSS CYCLE TYPE | MINIMUM EXPOSURE TIME FOR IUSS |
|---|---|---|
| All Metal Items & Non-Lumened Devices |
Non-Porous Cycle | 3 Minutes @ 270°F (132°C) 3 Minutes @ 275°F (135°C) |
| Lumened Instruments or Mixed Materials (Rubber/Plastic) |
Porous Cycle | 10 Minutes @ 270°F (132°C) 10 Minutes @ 275°F (135°C) |
When would I use a Prevacuum IUSS Cycle?
Tip: Whether a prevacuum or gravity IUSS cycle is required, it is important to review the IFU for the medical device, the packaging or containment system and the sterilizer. If discrepancies are found among the IFUs, they must be reconciled before proceeding.
Prevacuum cycles use an active process to remove air and inject steam into the sterilizer chamber during the conditioning and exhaust phase. Both non-porous and porous items (including mixed materials and lumened/cannulated devices) can be sterilized in prevacuum cycles if appropriate time and temperature are used, following device manufacturer's written Instructions for Use (IFU). Prevacuum IUSS cycle exposure parameters are typically four minutes at 270°F (132°C) or three minutes at 275°F (134°C) depending on the IFU requirements. The Prevacuum IUSS cycle also uses a one-minute dry time during the exhaust phase to decrease the steam inside the chamber before opening the door. Today this type of IUSS Cycle is preferred for more efficient air removal and steam penetration and to comply with device manufacturer's written Instructions for Use, so we will mention it in this article even though the focus is gravity IUSS.
What are the industry standards for monitoring IUSS Sterilization Cycles?
Per ANSI/AAMI ST79:2017, validated biological indicators (BIs) within process challenge devices (PCDs) should be used at least weekly, but preferably every day the sterilizer is in operation. This standard also applies to Gravity Immediate Use Steam Sterilization (IUSS) Cycle monitoring. Specific facilities will have policies for the frequency of biological monitoring, which could be weekly, daily, or even every load. Facilities should consider having the same policy of frequency for biological monitoring in both sterile processing, and IUSS which may be performed near other departments such as the operating room, or labor and delivery.
How to monitor Gravity IUSS Cycles
Gravity IUSS cycles should be monitored by using biological indicators and a Type 5 or Type 6 chemical indicator (CI) strip placed within a containment device and ran in an otherwise empty chamber. While there are Process Challenges Devices available for prevacuum cycles, gravity cycles, and prevacuum IUSS cycles, there are no pre-assembled PCDs for gravity IUSS cycles. Users are required to construct their own PCD as per industry standards.
For routine monitoring of gravity-displacement cycles, a representative of the same type of tray to be routinely processed by gravity-displacement cycles should be selected to serve as the PCD. Each type of tray configuration routinely used for gravity-displacement cycles should be tested separately. The BI and CI should be placed in the most difficult-to-sterilize portion of the PCD.
A Gravity IUSS PCD consists of a container validated for gravity IUSS, a BI validated for gravity IUSS, and a Type 5 or 6 CI. The containment device should be representative of the same type of tray routinely used in these cycles. The BI and CI are placed within the containment device creating the "process challenge device."
| STERILIZATION CYCLE | PROCESS CHALLENGE DEVICE |
|---|---|
| Prevacuum: Terminal Prevacuum: IUSS |
|
| Gravity: Terminal | |
| Gravity: IUSS |
Validated IUSS Container |
Remember: Do not place a preassembled PCD within a containment device. These are already manufactured in a self-containment device.
After the cycle is run, all sterility assurance monitors including BI, CI, and cycle parameters printout must show passing conditions and be reviewed by a qualified technician and all facility policies followed to confirm the sterilization process is complete and acceptable.
To learn more about monitoring gravity IUSS cycles, review product information available for the Celerity™ 20 STEAM BI for IUSS.
Earn FREE CEs and learn more about IUSS best practices with this STERIS University's Course: "Best Practices in Immediate-Use Steam Sterilization"
References:
- Association of periOperative Registered Nurses (2017). Guidelines for perioperative practice.
- Association of the Advancement of Medical Instrumentation (2017). ANSI/AAMI ST79:2017 Comprehensive guide of steam sterilization and sterility assurance in healthcare facilities.