Liquid Chemical Sterilization
What is Liquid Chemical Sterilization?
Liquid chemical sterilization (LCS) is used to liquid chemically sterilize heat-sensitive, immersible, reusable medical devices. When a device is liquid chemically sterilized, it is completely immersed in an active sterilant solution for a prescribed period of time at a controlled temperature and concentration. Validation of efficacy requires demonstration of at least a 6-log reduction of the most resistant organisms: bacterial spores. This type of processing makes liquid chemical sterilization in STERIS's SYSTEM 1E® Liquid Chemical Sterilant Processing System or SYSTEM 1® endo Liquid Chemical Sterilant Processing System an ideal solution for heat sensitive semi-critical medical devices such as duodenoscopes, bronchoscopes and other high-risk endoscopes.

Liquid Chemical Sterilization of Medical Devices
Liquid chemical sterilant processing of medical devices in a STERIS liquid chemical sterilant processing system requires three critical variables that are provided and controlled by the processor:
- Concentration of S40® Sterilant Concentrate (i.e. peracetic acid) – The peracetic acid-based sterilant is maintained above the validated minimum recommended concentration. Though peracetic acid is the active ingredient, the proprietary solution generated within the processor operates at a near neutral pH which provides a high degree of compatibility with sensitive medical devices.
- Exposure Time – The device is exposed to sterilant solution for 6 minutes.
- Temperature – During exposure, the solution is held at 45.5 - 60 degrees Celsius.
At the completion of the cycle, the processed device is removed from the liquid chemical sterilant processing system. At this point, the device is ready for transportation and immediate use. If not used immediately, the processed scope should be dried and stored in the same manner as advised by the scope manufacturer for a high-level disinfected scope. Scopes to be used for critical applications (SYSTEM 1E LCSPS only) must be processed immediately prior to use.
"If not used immediately, the processed scope should be dried and stored in the same manner as a high-level disinfected scope..."
Autoclaves, or steam sterilizers, are used to sterilize devices that are not heat-sensitive. A steam sterilizer uses time, psi (pressure), and 95-98% steam saturation to sterilize devices. Stainless steel surgical instruments can tolerate high temperature steam sterilization, while many endoscopes and other heat-sensitive semi-critical devices would be damaged. In comparison, SYSTEM 1E and SYSTEM 1 endo Processors offer high compatibility with sensitive device materials.
Phases of Liquid Chemical Sterilization in a SYSTEM 1 endo Processor

After placing a semi-critical device into a SYSTEM 1 endo Processor, attach device-specific Quick Connects and add a chemical indicator. Then, load the single-use S40 Sterilant Concentrate cup and start the cycle. Once a cycle is in process, the control screen will show the remaining time of the entire cycle as well as what phase the processor is currently in.
The phases of the SYSTEM 1 endo Liquid Chemical Sterilant Processing System are:
- Inflate Seal – Ensures the chamber is sealed
- Water Purge – Circulates water until the required temperature
- Water Fill – Chamber fills with the filtered water
- Warm/Mix – Creates the S40 Sterilant Use Dilution and heats to 46 degrees Celsius
- Exposure – Exposes the device load to S40 use dilution (the sterilant)
- Drain – Removes sterilant from chamber
- Rinse 1 – Rinses the device with filtered water
- Rinse 2 – An additional rinse with filtered water
- Air Purge – Removes excess water from channels of the device
- Deflate Seal – Allows user to open the lid of the system
Benefits of Liquid Chemical Sterilization
Liquid chemical sterilization provides a higher level of microbial kill assurance for heat-sensitive medical devices compared to high-level disinfection. SYSTEM 1E and SYSTEM 1 endo Processors offer a short cycle for easy turnaround of devices which may increase flow and throughput in a gastrointestinal (GI), bronchoscopy, urology, or surgical processing location. In comparison, other chemicals on the market that have clearance as a sterilant for medical devices would need to soak for two to 12 hours, depending on the product, to accomplish what a liquid chemical sterilant processing system can accomplish in 18 to 23 minutes.
An alternative is to process these heat-sensitive devices through ethylene oxide (EO) to achieve sterilization. This process has the advantage of providing a device that is terminally sterilized and can be stored, with the disadvantage that the process can take an entire day for one to two scopes.
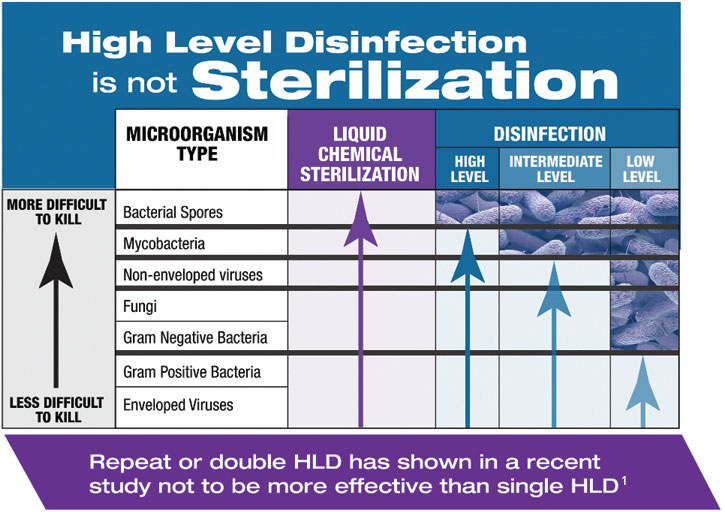
The Spaulding classification system recommends sterilization for semi-critical medical devices. By utilizing SYSTEM 1E or SYSTEM 1 endo Processor, many devices that previously were high-level disinfected because they could not withstand steam sterilization can now be liquid chemically sterilized. This is important in areas of the body where the body cavity is sterile, such as during an endoscopic retrograde cholangiopancreatography procedure (ERCP).
Myths of Liquid Chemical Sterilization
Liquid chemical
sterilization is a
form of high-level disinfection (HLD): |
There is no way to
challenge the system's
microbiocidal activity: |
Users will need to perform
quality testing of the
chemical indicator: |
| FALSE |
FALSE |
FALSE |
| Liquid chemical sterilant processing systems provide a higher level of microbial lethality compared to HLD. |
STERIS liquid chemical sterilant processing systems can be tested using a spore test strip. This strip checks for sporicidal activity of the sterilant solution during the cycle. |
The strips check for peracetic acid concentration, and quality testing is not required for single use, pre-portioned chemistry that goes into a closed system. |

Types of Chemicals Used in Liquid Chemical Sterilization
The active chemical used in STERIS liquid chemical sterilant processing systems is 35% Peracetic Acid. What is Peracetic Acid? Peracetic acid is an oxidative chemistry that denatures proteins, disrupts cell wall permeability and oxides sulfhydryl and sulfur bonds in proteins, enzymes and metabolites, which in the end shuts down cell function. This allows this chemical to kill or inactivate microbes on medical devices. While peracetic acid is an aggressive acid, when combined with a buffering system it can quickly kill microorganisms but maintain a neutral pH, giving it a high material compatibility rating. Find the MSDS for S40 Sterilant Concentrate peracetic acid chemistry here.
With any chemical sterilization process, temperature, time and concentration are critical to achieving sterilization. STERIS Liquid Chemical Sterilant Processing Systems provide fast cycles times of 23 minutes or less and can process a broad range of instrumentation. Other products on the market such as aldehydes are fixatives while the peracetic acid used in liquid chemical sterilization is an oxidative chemistry. Some products on the market have combinations of chemicals such as alcohols and phenolics or aldehydes. These types of chemicals may take several hours to achieve device sterilization, and most are labeled only for use as a high-level disinfectant.
Accessories for SYSTEM 1E and SYSTEM 1 endo liquid chemical sterilant processing system
The Sterilant® Chemical Indicator for S40®VERIFY is designed for use with STERIS liquid chemical sterilant processing systems. This CI gives a visual check to the end user to confirm that the minimum recommended concentration of peracetic acid is present in the sterilant use dilution. It displays a simple color change from blue to pink.
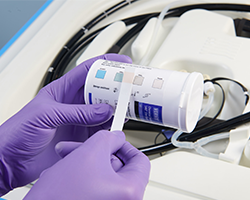
The VERIFY® Spore Test Strip for S40® Sterilant can be used to confirm spore kill in the STERIS liquid chemical sterilant processing systems. It is placed into a small clip and is processed through the cycle. At cycle completion, aseptic technique should be used to carefully place the spore strip into the provided media vial. The vial containing the strip is incubated for 24 hours and then read for growth or no growth.
 United States
United States
 Canada (EN)
Canada (EN) Canada (FR)
Canada (FR) Deutschland
Deutschland Italia
Italia United Kingdom
United Kingdom Australia
Australia New Zealand
New Zealand Singapore
Singapore Brasil
Brasil México
México






