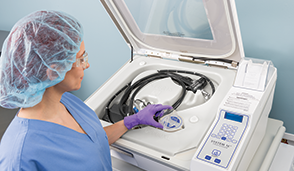
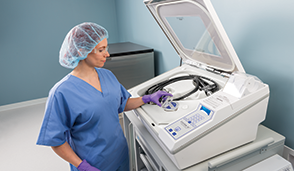




SYSTEM 1E™ Liquid Chemical Sterilant Processing System
- Validated Cycle: Liquid Chemical Sterilization in a rapid 23-minute cycle time
- Devices: Cleaned, reusable heat-sensitive critical and semi-critical medical devices
- Features: Liquid chemical sterilization which eliminates all viable microbial life, including bacterial spores such as C-Diff
Product Overview
 "Effectiveness of the SYSTEM 1E Liquid Chemical Sterilant Processing System for reprocessing duodenoscopes"*
"Effectiveness of the SYSTEM 1E Liquid Chemical Sterilant Processing System for reprocessing duodenoscopes"*
*American Journal of Infection Control (2016)
SYSTEM 1E&trade Liquid Chemical Sterilant Processing System
The SYSTEM 1E&trade Liquid Chemical Sterilant Processing System is for safe and effective reprocessing of heat-sensitive critical and semi-critical medical devices and is a practical solution for low temperature liquid chemical sterilization of complex flexible endoscopes, such as duodenoscopes used in endoscopic retrograde cholangiopancreatography (ERCP) procedures.
How SYSTEM 1E™ Liquid Chemical Sterilant Processing System Works
Liquid chemical sterilization enables rapid, efficacious reprocessing for cleaned, reusable, immersible, and heat-sensitive critical and semi-critical medical devices. The system utilizes S40® Sterilant Concentrate to eliminate all microbial life, including bacterial endospores. The S1E processor, two pre-filters, UV irradiation and a MaxPure™ filter work in harmony with S40 Sterilant Concentrate to provide a validated process for critical and semi-critical, heat sensitive devices. The gentle use-dilution protects delicate surgical instruments, including multi-channel flexible surgical endoscopes. The use-dilution is neutral pH, and rinses safely down the drain. Quick Connect adapters are designed to leak to ensure sterilant use dilution reaches all surfaces and angles of the device ports.
Why SYSTEM 1E™ Liquid Chemical Sterilant Processing System?
The SYSTEM 1E processor will help you process your devices with confidence. Its compact footprint and rapid 23 minute cycle time makes it convenient and ideal for a variety of locations. Compared to high-level disinfection, it provides a higher level of reprocessing assurance for heat-sensitive critical and semi-critical devices such as flexible or semi-rigid endoscopes.
Device Compatibility Matrix
Device Compatibility Matrix
Confirm that your medical devices and accessories are compatible for processing with our Low Temperature Systems
SEARCH FOR MY DEVICES|
INSTRUCTIONS FOR USE
|
|
|---|---|
| Document # | Document Title |
| S40 STERILANT CONCENTRATE - S4000 PACKAGE INSERT | |
| VERIFY SPORE TEST STRIP FOR S40 STERILANT - INSTRUCTIONS FOR USE | |
| WATER SOFTENER - INSTRUCTIONS FOR USE | |
| CHEMICAL INDICATOR FOR S40 STERILANT - LCC016 IFU | |
|
SDS
|
|
|---|---|
| Document # | Document Title |
| S40 STERILANT CONCENTRATE | |
|
WALL CHARTS
|
|
|---|---|
| Document # | Document Title |
| VERIFY CHEMICAL INDICATOR FOR SYSTEM 1E AND SYSTEM 1 ENDO PROCESSORS | |
| SYSTEM 1E LIQUID CHEMICAL STERILANT PROCESSING SYSTEM WATER TREATMENT GUIDE | |
| SPORE KILL TESTING IN THE SYSTEM 1E LIQUID CHEMICAL STERILANT PROCESSING SYSTEM WALL CHART | |
|
BROCHURE
|
|
|---|---|
| Document # | Document Title |
| SYSTEM 1E C-DIFF LEAVE BEHIND | |
|
SELL SHEET
|
|
|---|---|
| Document # | Document Title |
| SYSTEM 1E SELL SHEET | |
| S1E ULTRASOUND TRAY SELL SHEET | |
|
TECH DATA SHEET
|
|
|---|---|
| Document # | Document Title |
| SYSTEM 1E LIQUID CHEMICAL STERILANT PROCESSING SYSTEM | |
|
OPERATORS MANUAL
|
|
|---|---|
| Document # | Document Title |
| SYSTEM 1E OPERATOR MANUAL | |





