Knowledge Center
June 23, 2017
Hydrogen Peroxide Sterilization
What is Hydrogen Peroxide Sterilization?
Hydrogen peroxide sterilization, also known as hydrogen peroxide gas sterilization, is a low temperature sterilization process commonly used to sterilize heat-sensitive devices. A hydrogen peroxide sterilization cycle typically requires less time than alternative forms of sterilization, such as ethylene oxide sterilization. A hydrogen peroxide sterilization process involves H2O2 vapor filling the sterilizer chamber, contacting and sterilizing exposed device surfaces.
Once the sterilization cycle has completed, the vapor is vacuumed from the chamber and converted to water and oxygen.
Low Temperature Sterilization
Low temperature sterilization is a sterilization process best used for heat-sensitive devices that may be damaged by the conditions of a steam sterilization cycle. Ethylene oxide (EO) and vaporized hydrogen peroxide (VHP) are the two most common types of low temperature sterilization. Unlike heat-stable instruments, heat and moisture-sensitive devices are not always compatible with all models of low temperature sterilizers. Read our complete guide to low temp sterilization.
Explore our V-PRO® Low Temperature Sterilization Systems
Vaporized Hydrogen Peroxide Sterilization
Hydrogen peroxide sterilization is also known as vaporized hydrogen peroxide sterilization or VHP. Healthcare facilities more commonly choose vaporized hydrogen peroxide sterilization over ethylene oxide sterilization as their low temperature sterilization system. This preference for VHP is reflected by the declining use of ethylene oxide sterilization systems in hospitals.
The familiarity of hydrogen peroxide in households provides users a sense of confidence with hydrogen peroxide as a non-toxic, environmentally safe solution.
No ventilation is necessary for the vaporized hydrogen peroxide sterilization process and VHP machines only utilize one utility – power. No extra water, steam, or compressed air utilities are necessary.
Hydrogen Peroxide Sterilization Process
The vaporized hydrogen peroxide sterilization process is as follows:
- Liquid H2O2 gets converted into vapor
- The vapor fills the chamber, contacting all surfaces and penetrating lumens
- After sterilization, the vapor is vacuumed from the chamber and converted into water and oxygen
Hydrogen Peroxide Gas Process (No Plasma)
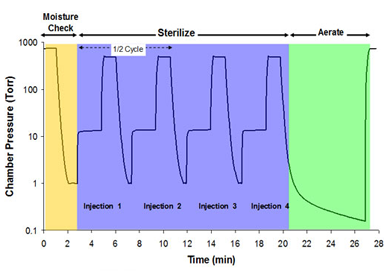
Hydrogen Peroxide Sterilization Guidelines
Both the Food and Drug Administration (FDA) and International Organization for Standardization (ISO)* require sterilizers to be safe and effective, and vaporized hydrogen peroxide sterilizers are no exception.1
Safety for the patient – Hydrogen peroxide sterilizers must follow ISO guidelines to ensure there are no toxic residues remaining on the devices that would be of concern for patients.
Safety for devices – Hydrogen peroxide is known for excellent material compatibility with a wide variety of materials.
Safety for staff – One of the most important safety aspects of gaseous sterilization is the assurance that the sterilizer is safe for the Sterile Processing Department staff. The Occupational Safety and Health Administration (OSHA) in the United States and other regulatory bodies for other countries have developed strict guidelines for hydrogen peroxide exposures. OSHA's Permissible Exposure Limit (PEL) for hydrogen peroxide exposure is 1ppm over an 8-hour Time Weighted Average (TWA).2 All VHP sterilizers must adhere to these guidelines as well as the OEM's Instructions for Use (IFU) to ensure the safety of the operator and SPD personnel.
Safety for environment – Because water and oxygen are the only by-products from a VHP sterilization process, this type of sterilization is not harmful to the environment.
Hydrogen Peroxide Sterilization Challenges
As with any form of sterilization, SPD personnel should be aware of challenges associated with vaporized hydrogen peroxide sterilization. Below are some of the challenges associated with vaporized hydrogen peroxide sterilization:
- Chamber size is typically smaller than that of steam sterilizers/autoclaves
- Sterilization cycles have specific device and load limitations based on design and manufacturer validation
- Unlike heat-stable instruments, heat and moisture-sensitive devices are not always validated or compatible with all models of VHP sterilizers; the Sterile Processing Department must confirm device validations for a VHP sterilizer
- Pre-processing of devices is critical (cleaning, drying, wrapping, etc.)
*The applicable ISO standard in this case is ISO 14937.
1 https://university.steris.com/resources/the-evolution-of-hydrogen-peroxide-gas-technologies-part2/
2 V-PRO Environmental H2O2 Safety Test