Knowledge Center
April 13, 2020
Your Guide to VHP Low Temp Sterilization

Tasks performed prior to sterilization influence the success of any sterilization process. Key steps taken during decontamination, preparation and packaging of materials for vaporized hydrogen peroxide (VHP) low temp sterilization must be followed. It's important to understand how these steps impact successful sterilization of medical devices.
Key steps include:
- Properly cleaning medical devices
- Ensuring devices are dry
- Preparing devices in validated packaging at a prep and pack workstation
- Ensuring devices are not too cold for processing
- Following best practices for load configuration
In this three-part series, we're going to break down the importance of these steps, while providing tips & best practices to aid in successful sterilization of medical devices.

Part 1: Cleaning & Preparing Devices
Why Cleaning is Important for VHP Sterilization
It is important to properly and thoroughly clean devices prior to sterilization to ensure sterilant can reach all parts of the device. In order to be sterilized, devices must be clean.
The science of VHP sterilization:
Hydrogen peroxide kills microbes by oxidizing amino acids and proteins. Any remaining soils, instrument cleaning chemistries and even water deposits act as a protective barrier that can hinder sterilant from reaching potentially harmful microbes.
To see how excess soil can hinder the sterilizer process, review this video which shows how tissue or blood left on instruments will break down the hydrogen peroxide into water and oxygen, which can affect the sterilization process.
As this video demonstrates, the tissues and blood rapidly break down hydrogen peroxide. The clean stainless-steel coupons show no reaction. The presence of residual blood and protein is creating a greater challenge to the sterilization system – the sterilant is being used to break the excess down blood instead of being used to sterilize the devices. essentially using up the sterilant. If there's too much residual blood or proteins on devices, proper sterilization cannot be achieved.
Why It Is Important To Dry Devices Before VHP Sterilization
Devices need to be both properly cleaned and dry for proper sterilization. This becomes an important step for both steam, and hydrogen peroxide (low-temp) sterilization.

Small moisture droplets can remain
in improperly dried devices
Water exposed to a vacuum process evaporates and becomes part of the air/gas mix in the sterilizer chamber. As it does, the pressure in the chamber rises. Most small amounts of moisture are evaporated. However, when there is an excessive amount of moisture, the pressure in the chamber rises too high and triggers the sterilizer to act as if air is leaking into the chamber, aborting the cycle.
But what if there's only a small amount of moisture?
Water trapped in constricted spaces, such as deep inside a lumen of an endoscope, may be difficult to evaporate. When exposed to a vacuum, this trapped water can freeze creating a physical barrier to sterilant penetration and potentially shielding bacteria.
Even worse, frozen water may condense leading to liquid hydrogen peroxide. Not only is liquid hydrogen peroxide less effective, but in rare cases, residual amounts can remain on devices. This is why it is always important to wear proper personal protective equipment (PPE) when unloading the sterilizer.
Continue to Part 2: Packaging for VHP Sterilization
Interested in Learning More and Earning Containing Education (CE) Credit?
Earn 1.0 CE Hour by learning more about the critical steps to ensure the best outcomes for hydrogen peroxide sterilization. Visit the STERIS University Course

Part 2: Packaging Instruments
Read Part 1: Cleaning & Preparing Devices
After instruments are inspected to ensure they are clean, dry, and properly functioning, they are then placed in a containment device for sterilization.

Peel Pouches

Wrapped
Sterilization Trays

Sterilization
Container Systems
Packaging being compatible with a sterilization process is not the same as being validated for a particular sterilizer.
It is critical the containment device and its accessories be validated for the sterilization process. This includes accessories such as corner protectors, filters and instrument holders or organizers.
Being compatible with a sterilization process is not the same as being validated. For example, a silicone container may be compatible with vaporized hydrogen peroxide because of the material composition. However, it may not be validated for use in the VHP sterilization cycle being run.

When packaging instruments for sterilization, they should be placed in such a way to promote sterilant contact with all surfaces. This means devices should be held in an open position so sterilant can access restricted spaces, and evenly dispersed throughout the tray with cords looped loosely. The instrument manufacturer's Instructions for Use (IFU) must be followed, including requirements to disassemble components of the device.
Trays are then enclosed within sterilization wrap which must be durable and non-linting. It too must be validated for the intended trays and sterilization process.
Sterilization pouches provide the most flexible option for enclosing instruments and instrument sets for hydrogen peroxide sterilization. STERIS's Vis-U-All™ Low Temperature Pouches have been validated for placement of instrument trays within a pouch. This means you can quickly pouch validated trays (such as PRO-LITE Sterilization Trays) without having to use any wrap. This allows for quick, and consistent, enclosing of trays which can then easily be transported while maintaining aseptic transfer to the Operating Room.
|
Pros |
Cons |
|
|
Wrapping |
|
|
|
Pouching |
|
|
Be sure to include necessary chemical indicators in the tray prior to pouching. Now that the sets are packaged, closed and labeled, they may move into the VHP sterilizer for sterilization assuming they're not too cold.
How Device Temperature Affects VHP Sterilization
Temperature of devices is an important factor in the VHP sterilization process because instrument sets that become too cold can lead to the condensation of vaporized hydrogen peroxide.
You may be wondering how instrument sets could get that cold. While it varies, the primary cause is the ventilation systems. When devices are placed beneath an air conditioning vent the cooled air blowing onto tables and devices may be cooler than the overall room temperature. This difference can cause condensation on cold metal instruments. Excess moisture caused by condensation can cause a cycle to abort or create a shield from sterilant.
While problems from this are uncommon, it's important to be aware and ensure loads are not too cold before entering the sterilizer.
Continue to Part 3: Cycle & Load Configuration
Interested in Learning More and Earning Containing Education (CE) Credit?
Earn 1.0 CE Hour by learning more about the critical steps to ensure the best outcomes for hydrogen peroxide sterilization. Visit the STERIS University Course

Part 3: Cycle & Load Configuration
Read Part 1: Cleaning & Preparing Devices
Read Part 2: Packaging Instruments for Low Temperature Sterilization
There are two important considerations when loading items for sterilization.
- Cycle and Device Limitations
- Load Configurations
Always follow manufacturer recommendations of both the sterilizer and devices.
Cycles and Device Limitations
Each cycle of a VHP low-temp sterilizer is characterized by the type of devices that can be processed in that cycle. For example, the non-lumen cycles cannot be used to sterilize a lumened device or a flexible scope.
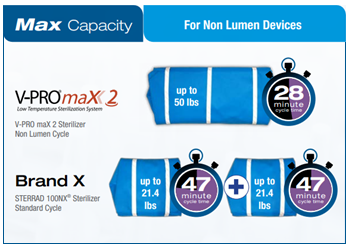
Sterilization cycles may also impose limits on the number of devices that can be processed within a single cycle. The V-PRO s2 Flexible cycle can process 1 single or dual flexible scope*, with up to 11 lbs. of short stainless steel lumens or non-lumened instruments in a single cycle
Understanding the cycle limitations is just as important as the weight of the total load you're processing. Having too much weight within a sterilizer can have a negative impact on the ability to sterilize. This is true for both the total load weight and the individual weight limits on containers and pouches. While some sterilizers can only process around 21 lbs. of devices in a single cycle, other can process up to 50 lbs. Understanding the cycle limitations of your low-temp sterilizer is important to the success of your sterilization process.
Load Configurations
Items must be placed in the chamber in a way to ensure sterilant can contact all surfaces of the items. Trays and containers are placed flat on the shelf. They should not be placed on an edge unless specifically recommended by the containment device manufacturer. Always follow the sterilizer manufacturer recommendations for proper load configurations and restrictions.
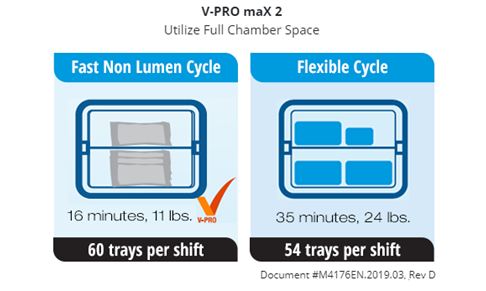
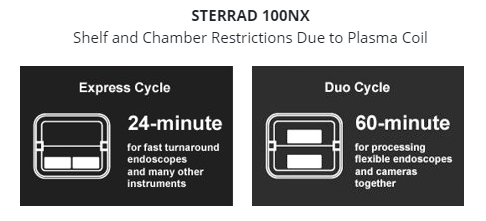
Additionally, hydrogen peroxide sterilizers that employ plasma utilize a plasma coil inside the chamber which reduces the amount of usable space. Items should not touch the plasma coil as contact may cause cycle aborts.
Preparation & Packaging for VHP Low-Temp Sterilization
The sterilization process is complex. Cleaning, rinsing, drying, testing and packaging of instrumentation can have a direct impact on the success of hydrogen peroxide sterilization. Take the time to review your cleaning, rinsing and drying procedures to ensure the best sterilization outcomes. Obtain, review and confirm that original equipment manufacturer (OEM) Instructions for Use of instruments, containment devices and sterilizers are followed when testing and packaging instrumentation for sterilization.
Interested in Learning More and Earning Containing Education (CE) Credit?
Earn 1.0 CE Hour by learning more about the critical steps to ensure the best outcomes for hydrogen peroxide sterilization. Visit the STERIS University Course
* Lumen ≥ 1mm and ≤ 990mm, please refer to the Operator Manual for detailed lumen dimension information and how to identify devices for loads.