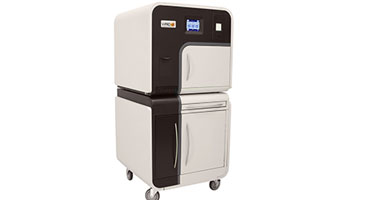

V-PRO™ 60 Low Temperature Sterilization System
- This product is no longer available for purchase in North America and has been replaced with the V-PRO s2 Low Temperature Sterilizer.
Product Overview
This product is no longer available for purchase in North America and has been replaced with a newer model. The V-PRO s2 Low Temperature Sterilizer can offer you more productivity and more flexibility with fast cycles, mixed loads and seamless connectivity to meet the demands of your SPD and OR.
Olympus®
| Device Type | Model |
|---|---|
| Bronchoscopes | BF, such as BF1T60 |
| Cystoscopes | CYF, such as CYF V2 |
| Pleuroscope | LTF, such as LTF VH1 |
Karl Storz®
| Device Type | Model |
|---|---|
| Ureteroscope | 11278AU1 |
| Rhino Pharyngoscope | 11101RP2 |
| C-Mac Intubation Fiberscope | 11302BD2 |
| Fiberscope |
Stryker®
| Device Type | Model |
|
HD Cameras |
1488-010-000 |
Medline®
| Device Type | Model |
|---|---|
| Sterilization Wraps | Various |
Cardinal Health™
| Device Type | Model |
|---|---|
| Sterilization Wraps | Various |
Halyard Health®
| Device Type | Model |
|---|---|
| Sterilization Wraps | Various |
Do you have a device from the following manufacturers? Visit the V-PRO Device Matrix to find out if your device can be reprocessed in a V-PRO Low Temperature Sterilization System.
- Aesculap
- Arthrex
- BK Ultrasound
- Brainlab
- Braun
- Cardinal Health
- Fisher & Paykel
- GE Healthcare
- Hitachi Aloka
- Intuitive Surgical
- Karl Storz
- Kimberly-Clark
- Medline
- Medovations
- Mizuho
- Olympus
- Philips
- Richard Wolf
- St. Jude Medical
- Stryker
- Teleflex
- Truphatek
- Volk
- WelchAllyn
|
INSTRUCTIONS FOR USE
|
|
|---|---|
| Document # | Document Title |
| AMSCO V-PRO CHAMBER CLEANING IFU | |
|
WALL CHARTS
|
|
|---|---|
| Document # | Document Title |
| GUIDELINES FOR PREPARATION, PACKING AND LOADING OF AMSCO V-PRO LOW TEMPERATURE STERILIZATION SYSTEMS (ENGLISH) | |
| GUIDELINES FOR PREPARATION, PACKING AND LOADING OF AMSCO V-PRO LOW TEMPERATURE STERILIZATION SYSTEMS (FRENCH) | |
| INTERPRETATION GUIDE FOR VERIFY INDICATOR TAPE | |
|
BROCHURE
|
|
|---|---|
| Document # | Document Title |
| V-PRO 60 DEVICE REFERENCE GUIDE | |
|
TECH DATA SHEET
|
|
|---|---|
| Document # | Document Title |
| AMSCO V-PRO 60 | |
| V-PRO 60 LOW TEMPERATURE STERILIZATION SYSTEM - FRENCH | |
| V-PRO 60 LOW TEMPERATURE STERILIZATION SYSTEM - JAPANESE | |

