




Cycle Log Forms
- Simplifies cycle documentation
- Log forms document pertinent cycle information for cleaning, sterilization, liquid chemical sterilization, and high level disinfection processing
- Assists in compliance with AAMI, AORN and other national standard's documentation requirements
Product Overview
Cycle documentation is made simple with cycle log forms. Each log sheet records pertinent cycle information for autoclave cycles, high-level disinfection, ultrasonic indicators, and more.
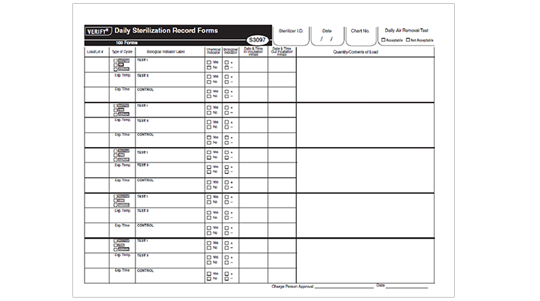
VERIFY® Daily Sterilization Record Forms (Product Number S3097)
- Documents Daily Air Removal Results (steam only), Daily Biological Indicator Test Results, Load Contents, Chemical Indicator Results and Biological Indicator Results
- Can be used for Steam, EO and VH2O2 Sterilization Processes
- Each double sided log form records five cycles and allows cycle printout to be affixed
- 3-hole punch for easy storage
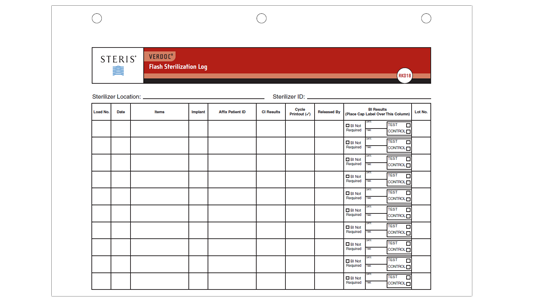
VERDOC® Immediate-Use Steam Sterilization (IUSS) Log Form (Product Number RK018)
- Documents Load Contents, Items Sterilized, Implant Information, Patient ID, and Biological Indicator Results
- 3-hole punch for easy storage
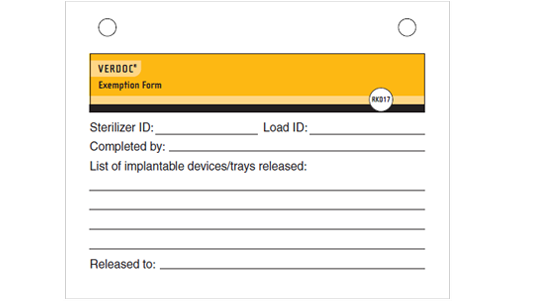
VERDOC® Exception Report (Product Number RK017)
- Complete documentation to comply with ANSI/AAMI ST79 early release documentation for implantable devices
- Each log sheet contains two exception reports
VERDOC VERIFY® All Clean Test Washer Indicator Log Form (Product Number RK029)
- Complete documentation to comply with ANSI/AAMI ST79 requirement of testing and documenting mechanical equipment for cleaning instruments
- Documents Washer ID, Location, Cycle No., Test Date, Pass/Fail, Follow up Actions
- Convenient adhesives to hold processed VERIFY All Clean Test Washer Indicator strips
- 3-hole punch for easy storage
VERDOC VERIFY® All Clean Test Washer Indicator and VERIFY RESI-TEST™ SWAB Cleaning Indicators Combination Log Form (Product Number RK030)
- Combines documentation of VERIFY All Clean Test Washer Indicator and VERIFY RESI-TEST SWAB Cleaning Indicators into one form
- Complete documentation to comply with ANSI/AAMI ST79 requirement of testing and documenting mechanical equipment for cleaning instruments
- Documents Washer ID, Location, Cycle No., Test Date, Pass/Fail, Follow up Actions for VERIFY All Clean Test Washer Indicator and Test Number, Technician, Instrument Type, and for failed results, actions taken for VERIFY RESI-TEST SWAB Cleaning Indicators
- Convenient adhesives to hold processed VERIFY All Clean Test Washer Indicator strips
- 3-hole punch for easy storage
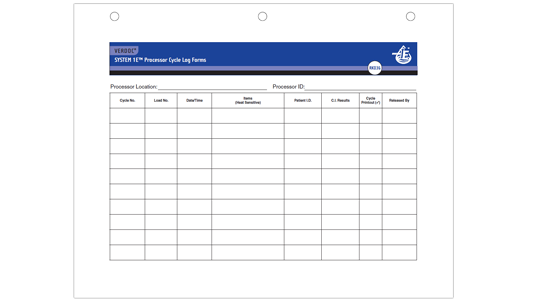
VERDOC® SYSTEM 1E Processor Cycle Log Forms (Product Number RK036)
- Documents Cycle No., Load No., Date/Time, Items processed, Patient ID and Test Results
- Double sided form records 24 cycles
- 3-hole punch for easy storage
VERDOC® Resert HLD Solutions Processing Log Form (Product Number RK038)
- Documents Patient ID, Date/time, Exposure time, Temperature, Items Processed and Test Results
- Double sided form records 22 cycles
- 3-hole punch for easy storage
VERDOC VERIFY® Ultrasonic Indicator Log Form (Product Number RK058)
- Complete documentation to comply with ANSI/AAMI ST79 requirement of testing and documenting mechanical equipment for cleaning instruments
- Log sheet documents Ultrasonic ID, Test Date, Pass/Fail
- Convenient adhesives to hold processed VERIFY® Ultrasonic Indicator strips
- 3-hole punch for easy storage
Shop Now
| Product Number | Description | Quantity | |
|---|---|---|---|
|
S3097 |
VERIFY® Daily Sterilization Record Forms |
4 pads/bx |
|
|
RK018 |
VERDOC® Immediate-Use Steam |
100 forms/bx |
|
|
RK017 |
VERDOC® Exception Report |
100 forms/bx |
|
|
RK029 |
VERDOC VERIFY® All Clean Test Washer |
100 forms/bx |
|
|
RK030 |
VERDOC VERIFY® All Clean Test Washer |
100 forms/bx |
|
|
RK036 |
VERDOC® SYSTEM 1E Processor |
100 forms/bx |
|
|
RK038 |
VERDOC® Resert HLD Solutions |
100 forms/bx |
|
|
RK058 |
VERDOC VERIFY® Ultrasonic |
100 forms/bx |
There are no literature documents found for this product.





