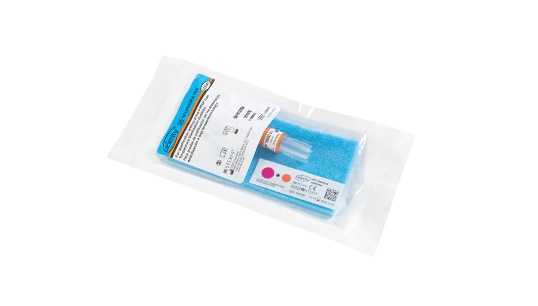


Celerity™ HP Challenge Packs
- Read Time: Provides biological indicator results in as little as 5 minutes
- Cycles: Used for qualification of V-PRO™ Low Temperature Sterilizers in less than three hours
- Feature: Challenge pack includes a Celerity™ HP Biological Indicator, a chemical indicator strip, and a blue polyurethane foam sheet
PRODUCT OVERVIEW
The Celerity™ HP Challenge Pack provides confidence for the initial use of your V-PRO Low Temperature Sterilization Systems after installation. With the Celerity™ HP Challenge Pack, only three consecutive cycles (most challenging) must be run in your V-PRO Sterilizer to qualify the sterilizer for same-day use. This means your V-PRO Sterilizer can be qualified and used in under three hours.
How the Celerity HP Challenge Packs Work
Each Celerity™ HP Challenge Pack includes a fast-read Celerity™ HP Biological Indicator, a Celerity HP Chemical Indicator strip, and a blue polyurethane foam sheet.
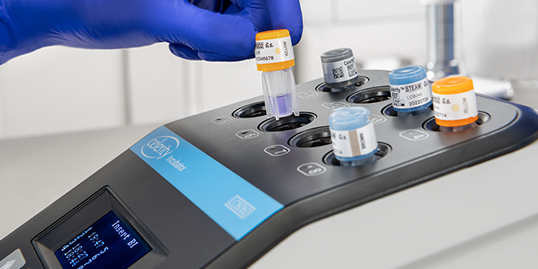
After processing, the BI is incubated using the Celerity™ Incubator, which provides a final read within 5 minutes (LCB059) or 20 minutes (LCB045) of incubation. A negative response from the incubator indicates that the BI underwent an acceptable sterilization process.
The self-contained BI reduces the risk of contamination and consequent false positives. Each BI vial is inoculated with Geobacillus stearothermophilus bacterial spores and contains a defined media within the vial cap. The vial cap label has a process indicator for hydrogen peroxide, which immediately provides confirms processing.
The chemical indicator strip is a Type 1 Process Indicator as defined in ANSI/AAMI/ISO 11140-1. This indicator provides immediate proof of processing. When exposed to sterilization conditions, the indicator ink on the chemical indicator strip undergoes a color change from magenta to orange/yellow (or lighter).
During sterilization, the pack must be exposed to a sufficient quantity of hydrogen peroxide to overcome the absorption of hydrogen peroxide by the polyurethane foam sheet and kill the bacterial spores within the self-contained biological indicator. The Celerity HP Challenge Pack provides a challenge to sterilization equivalent to or greater than that achieved by the worst-case sterilization load.
Why a Celerity HP Challenge Pack?
- Designed for Speed and Ease of Use – Speeds up the sterilizer qualification process by providing results within 5 minutes of incubation
- Safety and Compliance – Meets the requirements of AAMI ST58 Chemical sterilization and high-level disinfection in healthcare facilities
- Increased Productivity – Provides expedited and confident qualification of V-PRO Sterilizers, allowing the use of V-PRO Sterilizers the same day following installation or service
Celerity HP Challenge Pack Validated Sterilizers and Qualification Cycles
| Sterilizer | Sterilizer Qualification Cycle | Cycle Time |
|---|---|---|
|
V-PRO™ maX 2 Low Temperature Sterilizer |
Fast Non Lumen Cycle | 16 minutes |
|
V-PRO™ s2 Low Temperature Sterilizer |
Fast Cycle | 19 minutes |
| V-PRO™ maX Low Temperature Sterilizer V-PRO™ 60 Low Temperature Sterilizer V-PRO™ 1 Plus Low Temperature Sterilizer |
Non Lumen Cycle | 28 minutes |
| V-PRO™ 1 Low Temperature Sterilizer | Lumen Cycle | 55 minutes |
SHOP NOW
Browse a complete Item Number list and easily reorder Celerity™ HP Challenge Pack on Shop STERIS.
| Product Number | Description | |
|---|---|---|
| LCB059 | CELERITY 5 HP CHALLENGE PACK (5 TEST PACKS & 5 CONTROLS) - DOMESTIC | |
| LCB045 | CELERITY HP CHALLENGE PACK (5 TEST PACKS & 5 CONTROLS) - DOMESTIC | |
| LCB060 | CELERITY INCUBATOR |
|
INSTRUCTIONS FOR USE
|
|
|---|---|
| Document # | Document Title |
| CELERITY 20 P BI CHALLENGE PACK INSTRUCTIONS FOR USE | |
| CELERITY 5 HP CHALLENGE PACK INSTRUCTIONS FOR USE | |
|
TECH DATA SHEET
|
|
|---|---|
| Document # | Document Title |
| CELERITY 20 HP CHALLENGE PACK LCB047 | |
| CELERITY 20 HP CHALLENGE PACK (LCB045) | |
| CELERITY 20 HP CHALLENGE PACK LCB047 (FRENCH) | |
| CELERITY PCD_PCC082-PCC084-PCC085 TDS - ITALIAN (IT) | |
| CELERITY HP CHEMICAL INDICATOR CHALLENGE PACK - FRENCH (FR) | |
| CELERITY HP CHEMICAL INDICATOR CHALLENGE PACK - ITALIAN (IT) | |
| CELERITY HP CHEMICAL INDICATOR CHALLENGE PACK - SPANISH (ES) | |
| CELERITY HP CHEMICAL INDICATOR CHALLENGE PACK - PORTUGUESE (PT) | |
|
BROCHURE
|
|
|---|---|
| Document # | Document Title |
| V-PRO CONSUMABLES BROCHURE | |
|
SAMPLE PROCEDURES
|
|
|---|---|
| Document # | Document Title |
| QUALIFICATION TESTING OF V-PRO STERILIZERS FOLLOWING INSTALLATION OR MAJOR REPAIR | |



