PRODUCT OVERVIEW
The Celerity 5 HP Biological Indicator (LCB052) is a 5-minute rapid-read, single-use biological indicator designed to monitor vaporized hydrogen peroxide (VH2O2) sterilization processes. Also available is the Celerity 20 HP Biological Indicator (LCB044) with a 20-minute incubation time, both designed to help keep your operating room (OR) on schedule.
How the Celerity HP Biological Indicators Work
The biological indicator is self-contained, reducing the risk of contamination and false positives. Each biological indicator (BI) vial is inoculated with Geobacillus stearothermophilus bacterial spores and contains a defined media within the vial cap. The vial cap label features a process indicator for hydrogen peroxide sterilization.
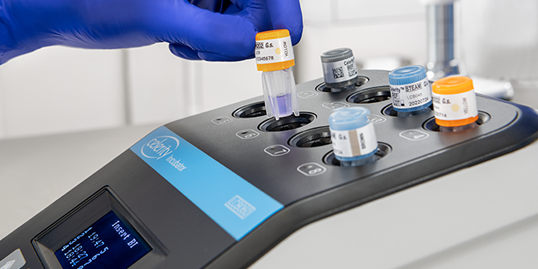
After a VH2O2 sterilization cycle, the process indicator on the vial cap will provide immediate confirmation of processing. The rapid readout biological indicator (BI) is activated using a twist and flick motion and does not require a separate activator. Upon activation, the BI is incubated using the Celerity™ Incubator. A negative response from the incubator indicates that conditions for sterilization were achieved.
Why the Celerity HP Biological Indicators?
Designed for Speed and Ease of Use
The Celerity HP Biological Indicator provides rapid results in as little as five minutes, so you can have confidence when releasing loads per facility policy after VH2O2 sterilization.
Increased Productivity
The Celerity HP BI expedites
cycle monitoring in a V-PRO or
STERRAD® Low-Temperature Sterilizer*1. Monitor
cycles with up to 50 lbs. of devices in less than 50 minutes when used in the Non Lumen
Cycle of the V-PRO maX or maX 2 Sterilizers.
Electronic Record Management with ConnectAssure Technology
Seamlessly connect and get immediate results from your Celerity Incubator
with ConnectAssure Technology. ConnectAssure Technology Data Export software
helps promote compliance throughout the facility by interfacing compatible
STERIS products with SPM Surgical Asset Tracking Software and many of the other leading instrument tracking systems.
* The Celerity HP Biological Indicators have been validated for use in all cycles on the STERRAD 100NX (with and without ALLClear Technology), STERRAD NX (with and without ALLClear Technology) and STERRAD 100S Sterilizers. Refer to the product’s instructions for use for complete details.
1 STERRAD is the Registered Trademark of ASP Global Manufacturing, GmbH