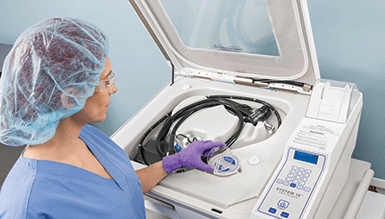
Liquid Chemical Sterilization
When compared to high level disinfection (HLD), liquid chemical sterilization (LCS) provides a higher level of reprocessing assurance for heat-sensitive critical and semi-critical medical devices.
STERIS's automated Liquid Chemical Sterilant Processing Systems are the most practical solution for low temperature liquid chemical sterilization of heat-sensitive complex endoscopes, including flexible multi-channel devices such as duodenoscopes used in endoscopic retrograde cholangiopancreatography (ERCP) procedures.
SYSTEM 1E Liquid Chemical Sterilant Processing System is intended for the liquid chemical sterilization of cleaned, immersible, and reusable heat-sensitive, critical and semi-critical medical devices.
SYSTEM 1 endo Liquid Chemical Sterilant Processing System is intended for the liquid chemical sterilization of cleaned, immersible, and reusable heat-sensitive semi-critical medical devices and their accessories.