Knowledge Center
September 16, 2021
Guide to Sterilization Pouches in Sterile Processing
What is a sterilization pouch and how is it used?
A sterilization pouch, or peel pack, is a disposable package used in a sterilizer to allow penetration of the sterilant to the items placed inside. After sterilization, these Class II Medical Devices maintain the sterility of the processed item.
A well-designed and correctly used sterilization pouch allows for effective sterilization, safe handling, and storage of all pouched items until needed for use. In addition to protecting items through this process, they also facilitate proper aseptic presentation of devices in an operating room. Much like the instruments they protect, sterilization pouches used in hospitals are also medical devices. Therefore, they require clearance by the Food and Drug Administration (FDA) and validation for use in specific sterilizers and cycles.
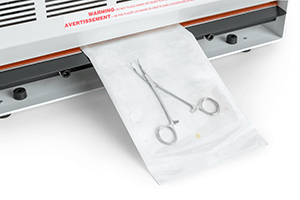
Sterilization Pouch being sealed in a heat sealer.
Sterilization pouches come in three designs:
- Self-seal pouches have an opening with an adhesive strip on one end. Items are placed into the pouch prior to sealing the open end with the adhesive strip.
- Heat-seal pouches have an opening on one end. Items are placed into the pouch prior to sealing the open end using a heat sealer.
- Rolls or tubing can be cut to the desired length and are open on both ends. Technicians will heat seal one end, place items into the pouch, and then heat seal the other end.
What are sterilization pouches made of?

There are two types of combination peel pouches:
- Paper/plastic – These pouches are for use in Steam or Ethylene Oxide (EO) sterilization
- Tyvek/plastic – These pouches are for use in Vaporized Hydrogen Peroxide (VHP) and EO sterilization.
A sterilization pouch consists of two main parts: medical grade paper, or Tyvek®, and a clear plastic film, held together by heat seal or with an adhesive. These materials are specifically designed to allow penetration of chemicals, heat, vapor, or steam.
It's important to remember that paper pouches are used in steam, and Tyvek is used in Vaporized Hydrogen Peroxide (VH2O2) sterilization, such as in V-PRO Low Temperature Sterilizers. Although they may look similar, when a pouch is used in the wrong sterilization process, a failure is likely to occur. Tyvek placed in steam will melt at higher temperatures. Paper placed in VH2O2 will absorb the sterilant vapors, which can hinder the sterilant from reaching the device. As well, paper that has absorbed VH2O2 can potentially damage instruments and can possibly catch on fire.
What are proper sterilization packaging guidelines?
Peel pouches are used for lightweight, low-profile instruments or medical devices. Per ANSI/AAMI ST79, the pouch should be of the right size and strength to accommodate the item(s) being packaged. For example, using paper/plastic pouches for heavy metal instruments could result in sterility maintenance problems due to events such as inadequate drying or rips and tears during storage or handling.
Validation/Testing

As mentioned earlier, Sterilization Pouches are Class ll Medical devices designed to allow sterilant penetration into the pouch, as well as maintain sterility of the device inside the pouch after sterilization. To validate that the pouch meets these requirements, pouches undergo rigorous testing to confirm their performance.
One crucial and stringent test performed during validation is half-cycle sterilization testing. This testing is used to simulate medical devices under worst-case conditions and confirm microbicidal efficacy. This test requires highly resistant bacterial spores to be placed in a challenging location to sterilize, such as inside lumens. The device is then pouched and sterilized in a cycle programmed with half of the cycle’s exposure time. All of the viable bacterial spores must be eliminated at the end of the half-cycle for the test to be considered a success.
How do you use sterilization pouches in a sterilizer?

- Choose the correct pouch for your sterilization modality: High Temperature or Low Temperature. As you choose your pouch, confirm the pouch materials are compatible with the medical devices to be packaged.
- Confirm the pouch is validated. The pouch and the device you’re sterilizing need to not only be compatible but validated for the sterilization process. Check the Instructions for Use (IFU) of the pouch or confirm validation with the manufacturer.
- Package Devices according to the IFU.
- Place the Pouch in the Sterilizer. Pouches can be placed flat on a shelf or placed on their standing edge facing the same direction, alternating paper or Tyvek to plastic.
Can you reuse sterilization pouches?
Sterilization pouches are single-use disposable devices and should not be reused. Any defects, tears, or damage also means the pouch should be discarded and not used for sterilization.
Can you double pouch sterilization pouches?
While there are no AAMI or AORN guidelines that state you must double peel pouch items for use in the Operating Room (OR), some ORs request certain items be double pouched to aid in aseptic presentation. Examples include several small items or instruments that might present a challenge during aseptic presentation.
When a pouch is validated by the manufacturer for double pouching, it is still important to review the pouch instructions for use. Make sure to follow your facility's policies and procedures when deciding whether to double pouch.
Tips for Double Pouching:
- The inner and the outer pouch should be film to film, both facing the same direction
- The inner pack should not be folded over
How to Choose the Right Sterilization Pouch
Considerations when selecting a sterilization pouch:
- Keep in mind the proper sizing and application of pouches to allow for adequate air removal, sterilant penetration, and drying
- Keep 1" of space around the item being sealed from all sides of the pouch
- Fill a pouch to a maximum of 75% of its packing volume to allow for the sterilant to penetrate the pouch and for the pouch to conform to the air evacuation process during sterilization
- Refer to manufacturer’s Instructions for Use for proper sizing and use of sterilization pouches
Tyvek® is a registered trademark of DuPont.
Contributors

Chasity Seymour
Clinical Education Specialist
![]()
Chasity Seymour is a Clinical Education Specialist with more than 15 years of Operating Room, Sterile Processing, Education, Management and Operational experience in healthcare. In five years managing Sterile Processing, she helped guide improvements of three departments including construction planning and workflow improvements.