





ASCENDO™ Submucosal Lifting Agent
- Quality Endoscopic Lift – Helps to clarify the margins of the target lesion and is designed to provide a submucosal cushion of height and duration
- Prefilled Syringe – Preparation is simplified in 5 ml or 10 ml offerings
- Biocompatible – After 28 days, there were no mortalities, signs of toxicity, pathological findings, and there were no macroscopic abnormalities attributed to the product1
- Sodium Hyaluronate – Commonly referred to as hyaluronic acid (HA), a biopolymer naturally present in the human body
Product Overview
The ASCENDO™ Submucosal Lifting Agent is intended for use in gastrointestinal endoscopic procedures. Submucosal lifting agents are used for the endoscopic lift of polyps, adenomas, early-stage cancers, or other gastrointestinal (GI) mucosal lesions before excision with a snare or endoscopic device.
HOW THE ASCENDO LIFTING AGENT WORKS
The effective removal of lesions during an endoscopic resection can be challenging and time-consuming. An injection needle delivers an endoscopic lifting agent or EMR lifting solution advanced through the endoscope's working channel. It helps to differentiate the muscle layer and submucosa, reducing the risk of perforation when endoscopic mucosal resection (EMR) is performed. Additionally, the blue coloring customary to EMR lifting solutions allows for delineation of the lesion margin compared to healthy tissue, thus supporting complete resection. Using a submucosal lifting agent can save physicians time by supporting quick localization of the separation of the polyp from the muscle wall as well as reducing risks like perforation.
The ASCENDO Submucosal Lifting Agent is designed to provide a submucosal cushion of height and duration, allowing the endoscopist a safe resection procedure. The submucosal lifting agent acts as a colored translucent solution to help clarify the area where it is injected. This helps to assist the endoscopist in visualizing the margins of the target lesion and performing the resection procedure, thereby decreasing the risk of damaging the external muscular layer, which could lead to perforation. The EMR lifting solution is injected into the submucosa beneath the lesion to be excised.
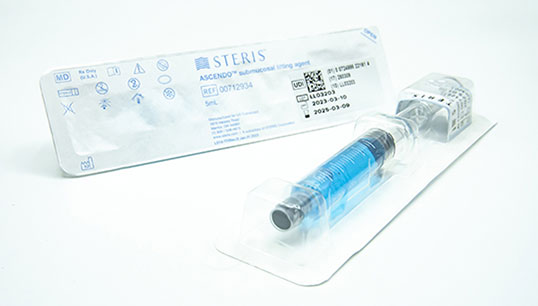
The ASCENDO Submucosal Lifting Agent is a solution used for the endoscopic lift of polyps, adenomas, early-stage cancers, or other gastrointestinal mucosal lesions before excision with a snare or endoscopic device in GI endoscopic procedures. It consists of sodium hyaluronate, Methylene Blue, and normal saline and is supplied in a ready-to-use, prefilled syringe containing 5 ml or 10 ml of the agent.
WHY THE ASCENDO LIFTING AGENT
- Quality Endoscopic Lift – Helps to clarify the margins of the target lesion and is designed to provide a submucosal cushion of height and duration
- Prefilled Syringe – Preparation is simplified in 5 ml or 10 ml offerings
- Biocompatible – After 28 days, there were no mortalities, signs of toxicity, pathological findings, and there were no macroscopic abnormalities attributed to the product1
- Sodium Hyaluronate – Commonly referred to as hyaluronic acid (HA), a biopolymer naturally present in the human body
1 Long-Term Implantation (28 Days) Combined with Assessment of Subacute Systemic Toxicity in Rats with Submucosal Injection Agent
Shop Now
| Product Number | Description | Volume per Syringe | Sterile | Unit of Measure | |
|---|---|---|---|---|---|
|
*This product is only available to International Customers |
|||||
| BX00712934 | ASCENDO™ submucosal lifting agent | 5ml | Yes | 10/box | |
| BX00712935 | ASCENDO™ Submucosal Lifting Agent | 10ml | Yes | 5/box | |
| BX00712934INT* | ASCENDO™ submucosal lifting agent | 5ml | Yes | 10/box | |
|
INSTRUCTIONS FOR USE
|
|
|---|---|
| Document # | Document Title |
| ASCENDO LIFTING AGENT - INTL IFU | |
| ASCENDO SUBMUCOSAL LIFTING AGENT - 10ML IFU | |
| ASCENDO LIFTING AGENT IFU | |
|
BROCHURE
|
|
|---|---|
| Document # | Document Title |
| POLYPECTOMY BROCHURE (US) | |
| POLYPECTOMY BROCHURE - CANADIAN | |
| POLYPECTOMY BROCHURE - FRENCH | |
|
CASE STUDY
|
|
|---|---|
| Document # | Document Title |
| ASCENDO CLINICAL SUPPORT DOCUMENT | |
|
QUICK START GUIDE
|
|
|---|---|
| Document # | Document Title |
| ASCENDO SUBMUCOSAL LIFTING AGENT QRG | |
|
SELL SHEET
|
|
|---|---|
| Document # | Document Title |
| ASCENDO SPEC SHEET - EN | |